Selection of a Macrocyclic GBCA: Barrow Neurological Institute’s Experience
Images
John Karis, MD, is the Director of MRI and Brain Imaging in the Department of Neuroradiology at Barrow Neurological Institute, part of Dignity Health St. Joseph’s Hospital and Medical Center, in Phoenix, AZ. In 2017, Dr. Karis and his team were tasked with selecting a macrocyclic gadolinium-based magnetic resonance imaging (MRI) contrast agent. Here we describe how the team went about making the selection, and their subsequent experience with that agent, ProHance (gadoteridol), since they made the switch.
Background
The first gadolinium-based contrast agent (GBCA) approved for use by the U.S. Food and Drug Administration (FDA) was the linear agent Magnevist (gadopentetate dimeglumine) in 1988, followed by the macrocyclic agent ProHance in 1992. Both Magnevist and ProHance are non–tissue-specific, extracellular fluid (ECF), multi-use agents that were initially approved for imaging the central nervous system (CNS). Since then, several additional GBCAs have been approved, and there are now 6 multi-use agents approved for use in the United States for a variety of indications.
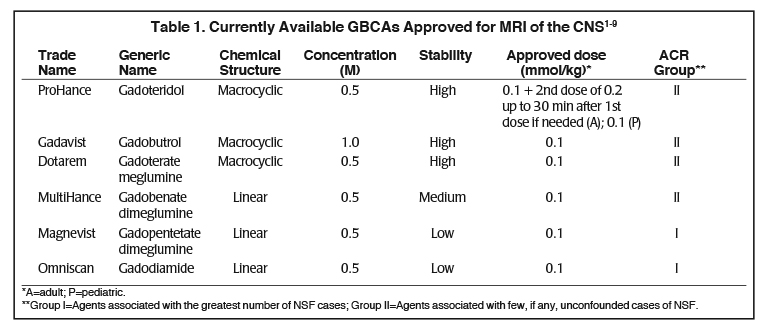
Several factors differentiate the 6 contrast agents approved for MRI of the CNS.1-9 (Table 1) In terms of physicochemical properties, they vary in chemical structure (macrocyclic vs linear), ionicity (ionic vs. non-ionic), and gadolinium concentration (0.5M vs 1.0M). In addition, all possess similar T1-relaxivity, with the exception of MultiHance (gadobenate dimeglumine), a high-relaxivity GBCA. ProHance, the macrocyclic agent that has been on the market longest in the U.S. is also the only agent approved at up to triple the standard 0.1 mmol/kg dose.
Stability, the strength with which the GBCA chelate holds on to the gadolinium ion, is another differentiator of the GBCAs: those with a macrocyclic chelate bind most tightly to the gadolinium and thus are characterized as high-stability. MultiHance, the high-relaxivity linear agent, is considered intermediate-stability, while the conventional-relaxivity, linear agents have the lowest stability, with the greatest propensity to release gadolinium, albeit in trace amounts with no indication of clinical consequences.7,9 (Table 1) In 2006, it was reported that the use of the linear GBCAs Magnevist, Omniscan, and OptiMARK (no longer available in the U.S.) was associated with the potential for development of nephrogenic systemic fibrosis (NSF) in patients with severe renal dysfunction. Although the pathogenesis of NSF is still unknown, at that time stability became a very important property of GBCAs, because the vast majority of unconfounded cases of NSF were associated to GBCAs with the lowest stability, especially linear nonionic.10 As a result, the American College of Radiology (ACR) stratified the GBCAs based on NSF risk: the macrocyclic agents and the high-relaxivity linear agent MultiHance were grouped together, and the risk of NSF with these Group II agents was considered to be “sufficiently low or possibly nonexistent,” such that patients did not require renal function screening prior to administration.9 (Table 1) To date, the vast majority of NSF cases have been shown to occur following administration of a low-stability, linear GBCA, and few unconfounded (single-agent) cases of NSF have been described following use of a macrocyclic agent. Among the macrocyclic agents, Gadavist has been associated with three unconfounded cases of NSF.11
More recently, a number of published findings showed gadolinium retention in the brains of patients who had received contrast in the past.12 Although such reports showed that gadolinium retention can be seen following administration of any of the GBCAs, all preclinical studies indicate greater levels of gadolinium retention with the linear agents than with the macrocyclic ones.13 Even among the macrocyclic agents, there seem to be differences in gadolinium retention: studies show ProHance is associated with less gadolinium retention in bone and brain compared to Gadavist.14,15 More significant for healthcare professionals, in patients with normal renal function, no harmful or adverse clinical effects have been associated with gadolinium retention in the brain or body.15 While the FDA has required the addition of precautionary statements regarding gadolinium retention in the GBCA product labels, importantly the FDA also noted that the clinical consequences of gadolinium retention have not been established in patients with normal renal function.16 Nevertheless, both NSF and gadolinium retention have prompted institutions that offer radiologic services to reevaluate the GBCAs they routinely offer as part of their clinical practice.
Barrow Neurological Institute
Barrow Neurological Institute, established in 1961, is a large, world-class neuroscience center with 8 clinical MRI scanners, including an intraoperative scanner, and an MRI research facility. The volume of MRI scanning at Barrow Neurological Institute is impressive, with approximately 95 MRI exams per day, and over 31,000 MRI exams per year. The MRI scanners at Barrow Neurological Institute are used to perform inpatient, outpatient, and emergency department (ED) diagnostic exams, with a minimum of two scanners running on a 24/7 basis. In addition, the magnets are used for surgical planning and intraoperative MRI, as well as for pulse sequence research and development.
Dr. Karis arrived at Barrow Neurological Institute in the early 1990s, soon after GBCAs were approved for use. Early on, Magnevist was used, followed by a switch to Omniscan (gadodiamide). In the late 1990s, due to a combination of early reports of NSF, as well as the higher relaxivity, Dr. Karis’ group switched to MultiHance, and continued to use MultiHance for a number of years. However, starting in 2014, several groups reported T1-weighted signal hyperintensity in the brains of patients on noncontrast scans (reviewed in McDonald, 2018), indicating gadolinium retention.12 As mentioned above, these signal hyperintensities could be found in patients that had received any GBCA, but appeared to be less pronounced with the macrocyclic GBCAs. As a result of these findings, as well as public concern around gadolinium retention, in January of 2017, Dr. Karis and colleagues decided to switch again, this time to a macrocyclic agent.
Switching to a Macrocyclic GBCA
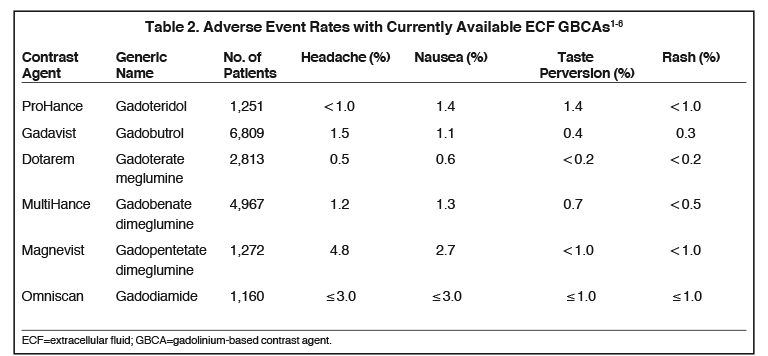
Dr. Karis had had some prior experience with Gadavist at another institution, so this was the macrocyclic initially selected; however, after a brief period, due largely to positive experience with Bracco contrast agents in X-ray and computed tomography (CT), and the potential advantages of standardizing system-wide with Bracco products, Dr. Karis decided to evaluate ProHance. Based on relaxivity, Dr. Karis trusted that the image quality would be there, so his focus was on patient tolerability. Specifically, before the switch could be made, it was necessary to ensure that in terms of patient safety, there would be no disadvantages. Dr. Karis approached the decision cautiously, deciding to administer ProHance to 100 patients in the outpatient imaging department to evaluate whether there was any increase in adverse events (AEs), specifically nausea and vomiting. Based on the Prescribing Information (PI), Dr. Karis knew that there were no reported differences in AE rates among the macrocyclic agents (Table 2), so he did not anticipate an increase. However, he wanted the technologists to feel comfortable knowing that the patients’ safety and comfort was paramount, and that their final selection of GBCA would be based on what was best for the patient.
Barrow Neurological Institute’s Experience with ProHance
Once the 100-patient ProHance study was complete, it was apparent that there was no increase in any AEs. At that point, Dr. Karis instructed that ProHance be rolled out facility-wide, and ProHance use was expanded to include inpatients and ED patients. ED patients often receive ProHance injection as a bolus, where one might anticipate an increased incidence of nausea, but no increase was seen. Dr. Karis reported that they now use ProHance routinely, keeping other agents on formulary only in the event that if a patient has an allergic-type reaction to one agent, they can then administer a different agent.
Considerations in Switching to ProHance
The facility’s MRI technologists required no special training prior to the switch. Dr. Karis shared the PI data with the technologists so they would know there was no scientific basis upon which to anticipate an increase in AEs with ProHance. Ultimately, the MRI technologists concurred that they did not see any change in the AE rate with ProHance in any of the imaging settings. By the end of 2017, Barrow Neurological Institute reported that they use ProHance almost exclusively (>95%) for all of their MRI examinations.
Barrow now uses Bracco contrast media and delivery products institution-wide for X-ray, CT, and MRI. As a result of this standardization, Dr. Karis’ group has the added benefit of considerable cost savings, which they can use elsewhere in the imaging department to upgrade equipment, bring on staff, or make other improvements.
References
- ProHance® (Gadoteridol) Injection, [prescribing information]. Princeton, NJ: Bracco Diagnostics Inc.; April 2018.
- Gadavist® (gadobutrol) injection [prescribing information]. Wayne, NJ; Bayer HealthCare Pharmaceuticals; April 2018.
- Dotarem® (gadoteric acid) [product information]. Bloomington, IN: Guerbet LLC; April 2018.
- MultiHance® (gadobenate dimeglumine) injection [prescribing information]. Princeton, NJ; Bracco Diagnostics Inc.; April 2018.
- Magnevist® (brand of gadopentetate dimeglumine) injection [prescribing information]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; April 2018.
- Omniscan™ (gadodiamide) injection [prescribing information]. Princeton, NJ: GE Healthcare; April 2018.
- Idée JM, Port M, Robic C, Medina C, Sabatou M, Corot C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging. 2009;30:1249-1258.
- Shen Y, Goerner FL, Snyder C, et al. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Invest Radiol. 2015;50:330-338.
- American College of Radiology (ACR) Committee on Drugs and Contrast Media. ACR Manual on Contrast Media, Version 10.3. 2018. Available at https://www.acr.org/Clinical-Resources/Contrast-Manual. Accessed October 22, 2018.
- Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-1108.
- Endrikat J, et al, Invest Radiol. 2016 51: 537-543.
- McDonald RJ, Levine D, Weinreb J, et al. Gadolinium Retention: A Research Roadmap from the 2018 NIH/ACR/RSNA Workshop on Gadolinium Chelates. Radiology. 2018:181151.
- Guo BJ, Yang ZL, Zhang LJ. Gadolinium Deposition in Brain: Current Scientific Evidence and Future Perspectives. Front Mol Neurosci. 2018 Sep 20;11:335.
- Murata N, Gonzalez-Cuyar LF, Murata K, et al. Macrocyclic and Other Non-Group 1 Gadolinium Contrast Agents Deposit Low Levels of Gadolinium in Brain and Bone Tissue: Preliminary Results From 9 Patients With Normal Renal Function. Invest Radiol. 2016;51:447-453.
- McDonald RJ, McDonald JS, Dai D, et al. Comparison of Gadolinium Concentrations within Multiple Rat Organs after Intravenous Administration of Linear versus Macrocyclic Gadolinium Chelates. Radiology. 2017;285:536-545.
- US Food and Drug Administration (FDA). FDA Drug Safety Communication: FDA evaluating the risk of brain deposits with repeated use of gadolinium-based contrast agents for magnetic resonance imaging (MRI). July 27, 2015. http://www.fda.gov/Drugs/DrugSafety/ucm455386.htm. Accessed October 22, 2018.