Magnetic resonance enterography in inflammatory bowel disease
Images
Inflammatory bowel disease (IBD) is a debilitating, chronic, inflammatory disease comprising two predominant pathologies: Ulcerative colitis (UC) and Crohn’s disease (CD). Ulcerative colitis is typically confined to the mucosa of the rectum and large bowel, while Crohn’s disease can affect any portion of the GI tract and is often transmural. Despite these differences, magnetic resonance enterography (MRE) plays an important role in the diagnosis of both conditions.
This article is accredited for one SA-CME credit. Visit appliedradiology.org/SAM2 for full SA-CME information.
Inflammatory bowel disease is more common in North America and Western/Northern Europe, and is thought to affect approximately 1.4 million people in the U.S., and as many as 2.5 million to 3 million in Europe.1 Both UC and CD are typically diagnosed in children or young adults, although CD is sometimes diagnosed in a smaller subset of patients between 60 and 80 years of age. The exact etiological pathways leading to IBD have not yet been fully elucidated, but are thought to be multifactorial. IBD, and particularly CD, is thought to result from an altered gut microbiome and altered immune reactivity, in addition to pro-inflammatory factors mediated by mesenteric adipocytes. Genetic and environmental factors appear to contribute additional elements that promote disease. Additionally, CD and UC are at times indistinguishable, and a diagnosis of IBD unclassified is often made.
The major unmet clinical need is lack of a gold standard diagnostic test; diagnosis is typically made by a combination of clinical features, findings on endoscopy and histopathology, laboratory abnormalities, and imaging. The symptoms of CD are often more variable depending on the location of GI involvement.2 Clinical manifestations of UC include diarrhea, hematochezia, tenesmus, and fecal urgency. Laboratory abnormalities are nonspecific but can demonstrate anemia, hypoalbuminemia, and elevation of C-reactive protein and erythrocyte sedimentation rate. Imaging in IBD has historically been aimed at assessing the portions of bowel that are inaccessible to endoscopy. Cross-sectional imaging with CT and MRI is increasingly being used to evaluate IBD due to their capacity to assess submucosal and deeper tissues of the bowel and to evaluate for extra-intestinal manifestations.
Review of diagnostic methods
Endoscopy
Ileocolonoscopy directly visualizes the mucosa and allows for direct tissue sampling, resulting in high diagnostic sensitivity for mucosal disease. Only a short segment of the terminal ileum may be accessible. Capsule endoscopy (CE) can allow visualization of the mucosa throughout the small bowel; however, it does not allow tissue sampling and is contraindicated in stenosis or obstruction. A recent meta-analysis of CE and MRE demonstrated that CE was superior to MRE for proximal small-bowel Crohn’s disease.3 Additionally, multiple studies have demonstrated that CE is more sensitive than MRE for detecting small aphthous lesions.4,5,6 It is known that MRE has relatively decreased sensitivity for mild disease restricted to the superficial mucosa. A screening program involving direct mucosal visualization by colonoscopy is commonly implemented for patients with pan-colitic UC, due to increased risk for colonic mucosal adenocarcinoma.
Medical imaging in IBD
Small-bowel follow through (SBFT) can evaluate the small bowel to detect stricture, fistula, and abscess. SBFT is relatively insensitive to mucosal disease and provides limited sensitivity for submucosal or deeper involvement. CTE and MRE have been shown to be superior to SBFT, particularly for detecting extra-enteric disease and complications.7
Ultrasound is noninvasive, does not impart ionizing radiation, and is generally tolerated by patients. Examination may be limited by luminal bowel gas, although this can be reduced by fasting and oral administration of intraluminal contrast.8 Although the ileocecal region and colon are often easily visualized, additional portions of the small bowel can be difficult to see due to overlying bowel loops. Compared to CTE and MRE, ultrasound poorly demonstrates the extent of abnormalities and demonstrates poor longitudinal comparison between studies.9
Computed tomography enterography (CTE) generally demonstrates greater availability and lower initial costs compared with MRE; however, the overall cost benefit remains incompletely evaluated. Studies of the sensitivity of CTE vs MRE for small-bowel pathology have shown mixed results,7,10-12 while at least one study has shown improved sensitivity of CTE for distinguishing perienteric features due to increased conspicuity of the mesentery on CTE.13 A major limitation of CTE is the cumulative ionizing radiation dose, especially in patients who would benefit from longitudinal imaging over their disease course.14
Magnetic resonance enterography
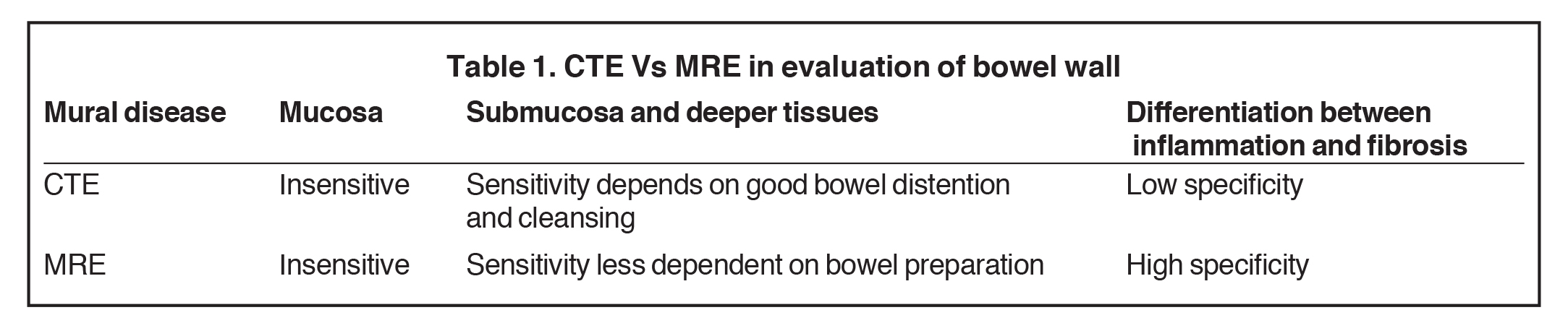
Magnetic resonance enterography demonstrates a greater ability to depict submucosal pathology compared to other diagnostic modalities. This includes determining the presence and extent of transmural inflammation, fibrotic disease, and other intra-abdominal complications. The modality also demonstrates improved ability to differentiate inflammation from fibrosis within the submucosa of the bowel wall and within the peri-enteric tissues (Tables 1 and 2).12,15,16 Additionally, MRE can demonstrate enteric and extra-enteric manifestations, including bowel obstruction, abscess formation, tethering, and fistulae.17-20 Finally, MRE is less dependent on bowel distention compared to optimal CTE.
Patient preparation for MRE
Various methods are available to prepare patients for MRE. The most commonly used agents include osmotic agents such as 2.5% mannitol, methylcellulose, or polyethylene glycol, which promote luminal distention by slowing down water absorption in the jejunum. Gastrointestinal prokinetic agents, such as erythromycin or metoclopramide, may be administered to speed gastric emptying.21,22 Recent research suggests that the sensitivity to bowel wall pathology related to CD is adequate even without bowel distention.15 At our institution, oral contrast use is not mandatory, and we rely on the high level of bowel wall contrast, achievable by MRE, to detect active or chronic IBD.
MRE acquisition
Multiple sequences are useful for bowel imaging. The breath hold 3-D gradient echo (3D GRE), T1W, and single-shot, T2W techniques are useful for bowel imaging.15 The high signal of diseased bowel becomes much more conspicuous if the adjacent fat is darkened by fat suppression.23 We use a multi-echo Dixon technique, which provides improved fat suppression with higher in-/out-of-plane resolution and contiguous bowel-segment imaging with improved contrast and edge sharpness. Fat-suppressed techniques are highly useful, and optimal fat suppression is achieved with spectral adiabatic inversion recovery (SPAIR).16,23 T1W images are acquired before and after gadolinium-based contrast is administered in the arterial, venous, and delayed phases. Longitudinal MRE evaluation is important to confirm improvement of active inflammation and to evaluate for the presence of unmasked chronic fibrotic disease.
Perfusion imaging has been shown to provide high diagnostic accuracy when combined with T2W images and postcontrast images.24,25 Perfusion imaging requires specialized scanning techniques or perfectly timed arterial, venous, and delayed phase enhancement, which raises the technical challenge of whole abdomen and pelvis imaging.
Diffusion weighted imaging is also used to improve sensitivity for detecting diseased bowel-wall segments and peri-enteric soft tissues.26,27 A recent study found improved sensitivity with DWI imaging in comparison with contrast-enhanced sequences for evaluation of inflammatory bowel disease.28
Image interpretation in Crohn’s disease
There are three subtypes of Crohn’s disease: Active inflammatory, fistulizing/perforating, and fibrostenosing.
Active inflammatory disease
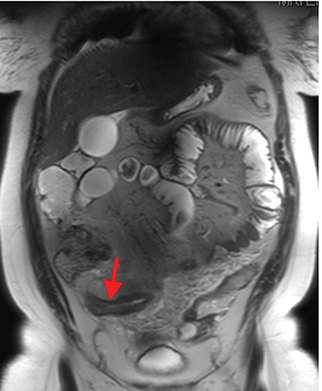
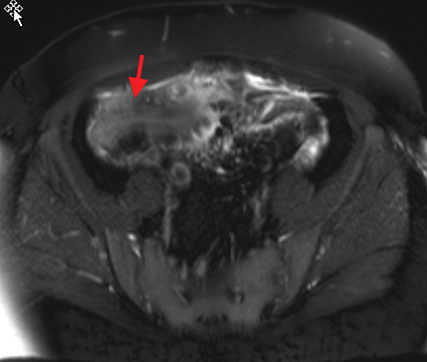
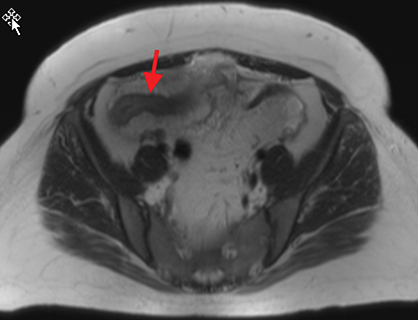
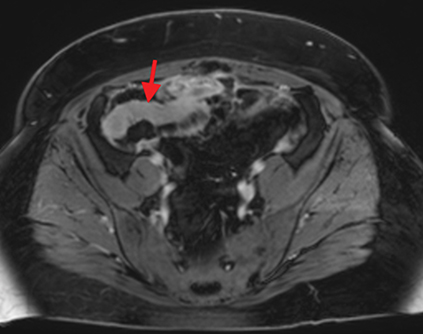
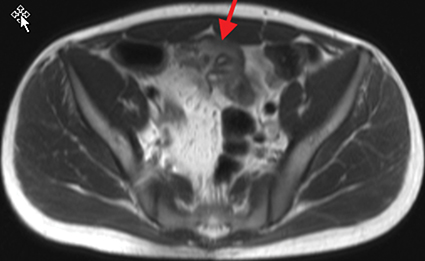
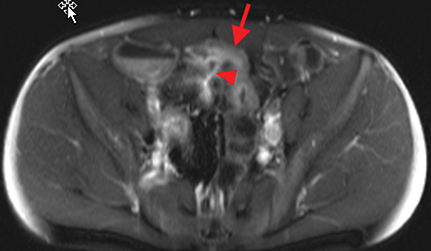
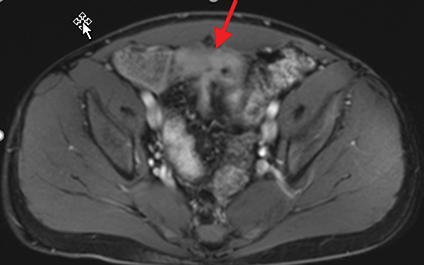
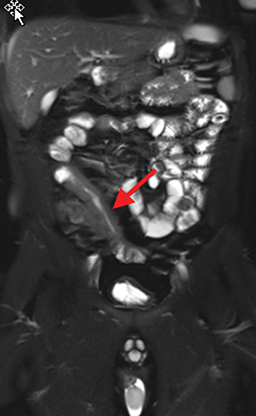
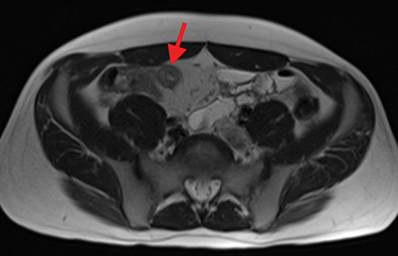
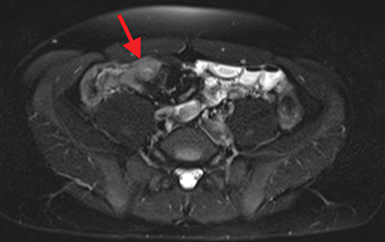
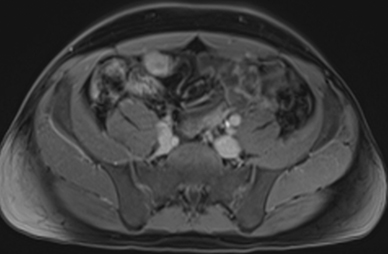
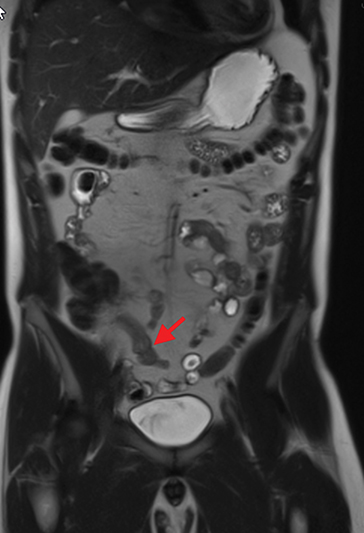
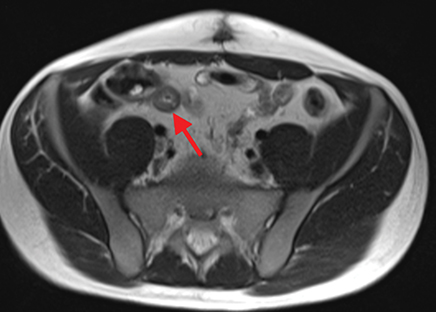
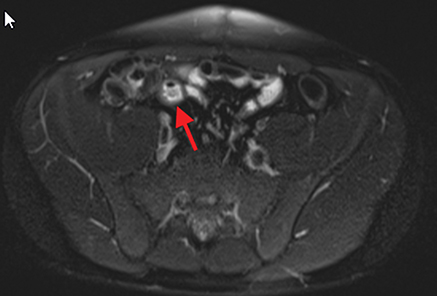
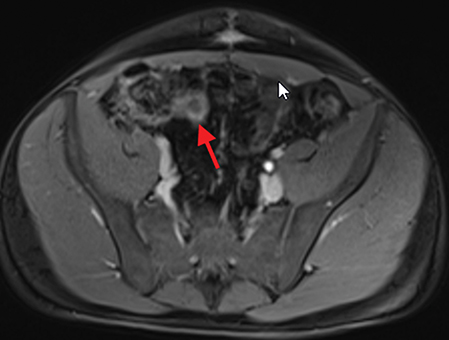
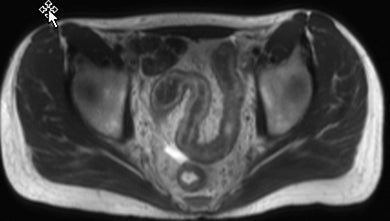
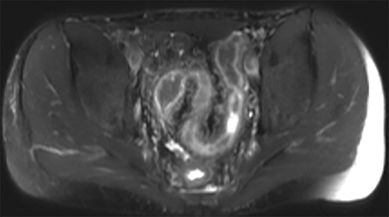
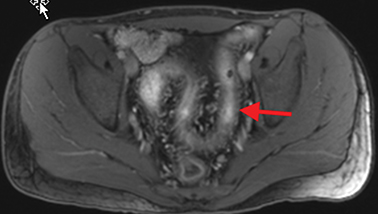
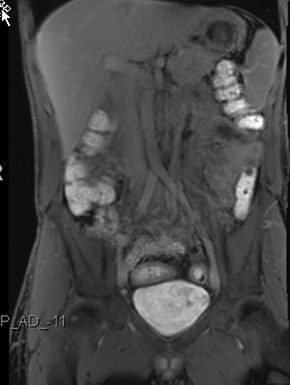
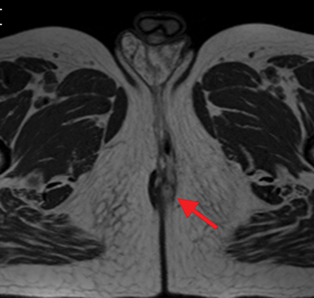
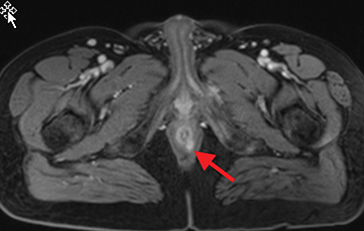
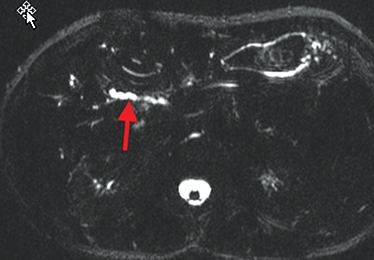
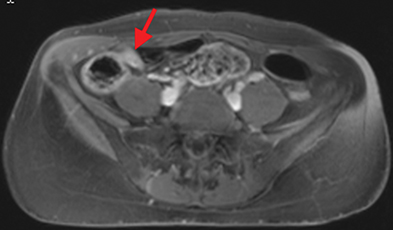
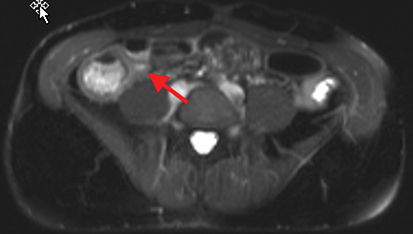
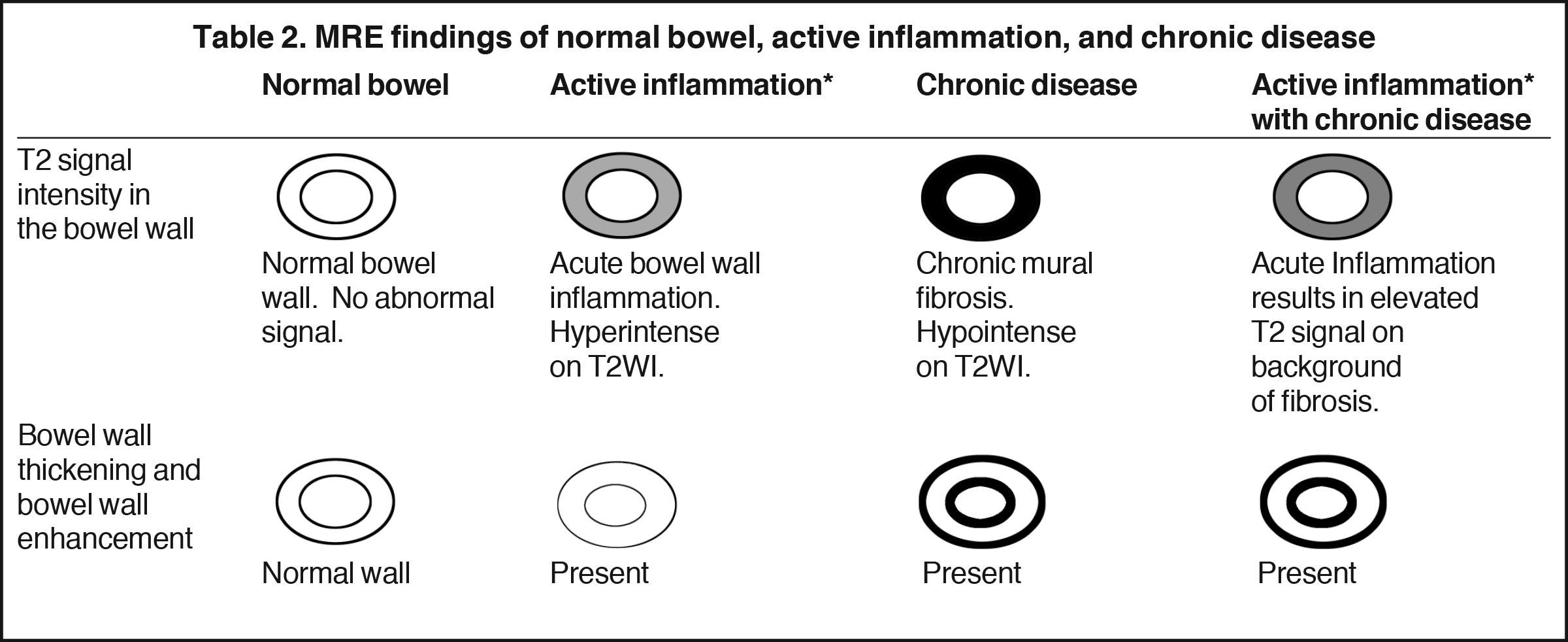
Active inflammatory Crohn’s is characterized by aphthoid and deep ulceration, wall thickening (greater than 4 mm), intramural and mesenteric edema, stratified enhancement pattern of the bowel wall, increased mesenteric vascularity (comb sign), and reactive lymphadenopathy.29 Active inflammation manifests as high signal intensity edema and inflammatory fluid on T2-weighted images that show enhancement on postgadolinium images coupled with bowel-wall thickening (Figures 1–3).
Chronic disease (fistulizing/perforating and fibrostenosing)
Chronic disease (fistulizing/perforating, and fibrostenosing subtypes) without active inflammation demonstrates low signal intensity fibrosis with possible stenosis and obstruction plus bowel-wall thickening and delayed enhancement on post-gadolinium imaging. The lack of T2W high signal intensity differentiates chronic disease from acute disease. Acute-on-chronic disease has features of both acute and chronic CD with increased signal on T2W images consistent with active inflammation, also with thickened bowel wall and retained contrast enhancement. Resolution of the elevated T2 signal is a marker of therapeutic change.
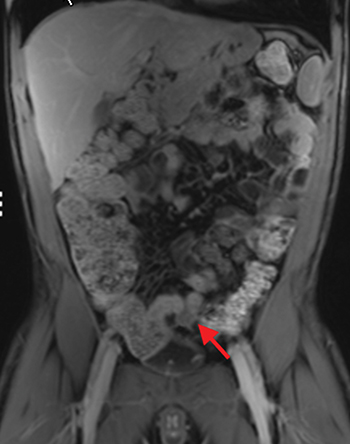
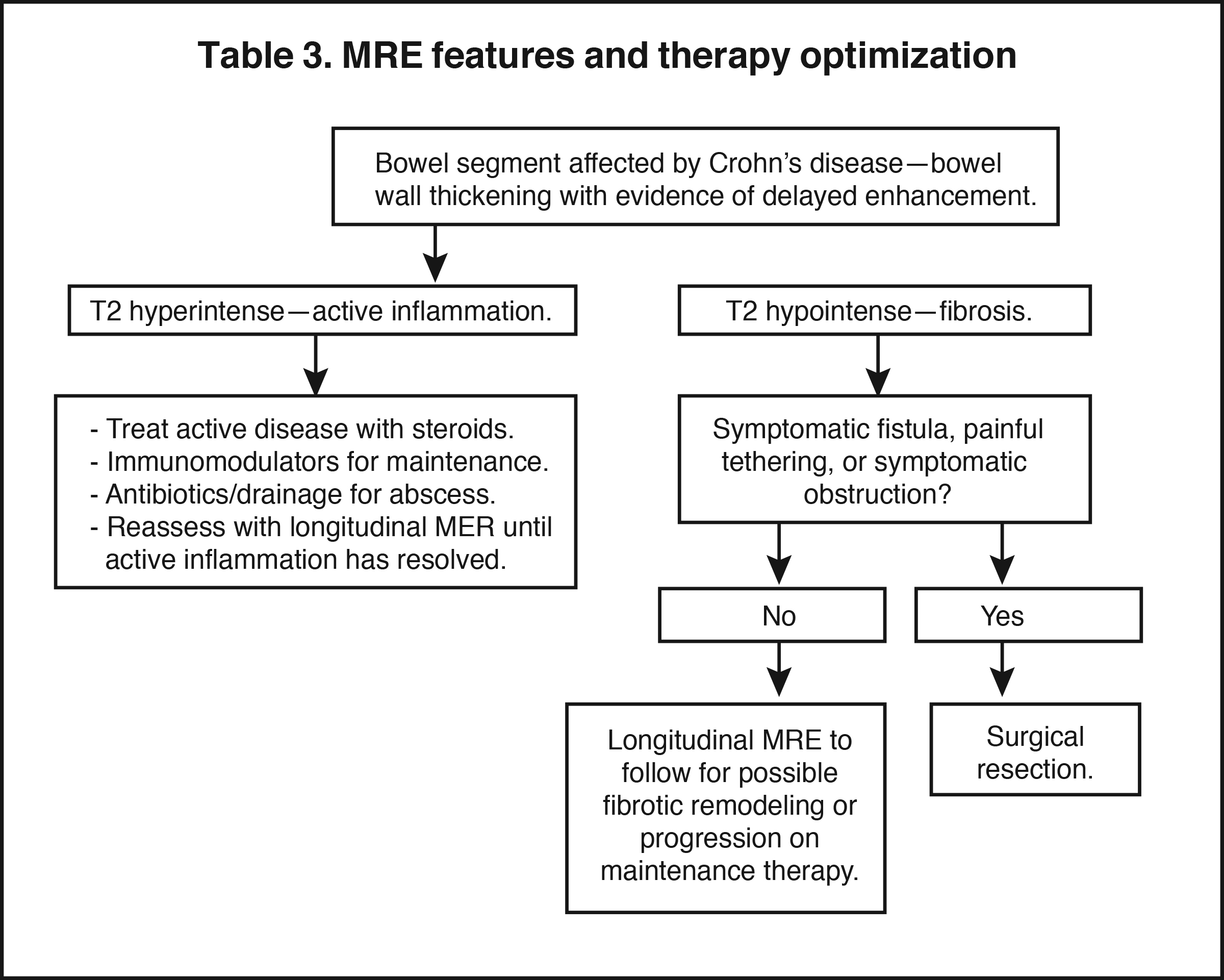
The fistulizing/perforating subtype is characterized by deeply penetrating ulcers that may lead to creation of a sinus tract, fistula formation, or abscess formation. The fibrostenotic subtype is characterized by bowel strictures and obstruction, which develop as a result of prolonged, chronic intestinal injury (Figures 2, 4, and Table 3).
Image interpretation in ulcerative colitis
Acute ulcerative colitis
Like Crohn’s disease, acute ulcerative colitis is characterized by increased T2 signal within the bowel wall. Normal colonic wall thickness is 2-3 mm; 8 mm has been reported in active cases (Figure 5).
Chronic ulcerative colitis
Chronic ulcerative colitis without active disease will manifest as wall thickening with mural fat deposition involving the rectum and and/or segments of large bowel. Additional findings include reduced distensibility, loss of haustra, and mural hyperenhancement. Contrast enhancement, edema, enlarged pericolonic lymph nodes, and engorged vasa recta (the comb sign) are all used as diagnostic indicators of active ulcerative colitis.30
Extra-enteric complications of Crohn’s disease
The extra-enteric complications consist of three major kinds:
Perirectal
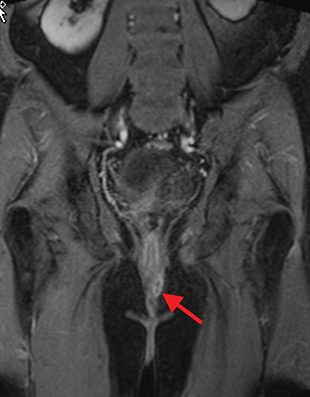
Perianal and perirectal abscesses are common in Crohn’s disease and are easily diagnosed with MRE (Figure 6).
Hepatobiliary
Hepatobiliary complications of Crohn’s disease include sclerosing cholangitis and mesenteric vascular thrombi formation. Magnetic resonance cholangiopancreatography (MRCP) is a heavily T2-weighted acquisition used to evaluate the biliary system. Classic beaded appearance of the intra- and extrahepatic bile ducts can be seen in sclerosing cholangitis on MRCP (Figure 7).
Musculoskeletal
Musculoskeletal disorders are a common complication of CD, with approximately 22% of patients developing joint inflammation.31 Complications include ankylosing spondylitis, avascular necrosis of the femoral head, or osteopenia; the latter two are often attributable to steroid therapy for active CD.
Clinical impact/outcome
MRE-based disease scores have recently been developed to quantify disease activity in Crohn’s disease. They generally include scoring of parameters such as mural thickness, mural T2 signal intensity, and avidity of contrast enhancement in comparison to an index tissue, such as normal bowel wall or psoas muscle. The Magnetic Resonance Index of Activity (MARIA) score and the CD MRE Index (CDMI) score have both been developed to use an adequate independent external reference standard and have been successfully validated in independent patient cohorts. Both MARIA and CDMI have high sensitivity (80-90%) for detecting active disease and high reproducibility between radiologists.32 A recent meta-analysis comparing CT and MRI to evaluate diagnostic accuracy in Crohn’s disease found no significant difference between the two modalities.33
Multiple studies comparing MR with colonoscopy as the gold standard have been performed in patients with UC. A recent study by Campari et al demonstrated good sensitivity (94%) but poor specificity (64%) for detecting actively inflamed colonic segments compared with ileocolonoscopy with biopsies.34 Another study by Ordás et al demonstrated a sensitivity of 87% and a specificity of 88% for detecting disease activity in ulcerative colitis using endoscopy as the reference standard.35
Limitations of MRE and solutions
The availability of MRE expertise and access may represent a relative limitation compared to CTE or SBFT. While bowel paralytics may reduce image deterioration on MRE, other sources of patient motion, particularly breathing, may be problematic. Newer MRE acquisition methods are under development to mitigate the complexity of patient-imaging techniques, while also overcoming image artifacts resulting from patient motion. The safety and avoidance of ionizing radiation, in a mostly young patient population with chronic-relapsing disease, favors routine use of MRE.
Conclusion
Magnetic resonance enterography generates reproducible, high-quality examinations of the small and large bowel with excellent sensitivity and specificity for inflammatory bowel disease and their complications. Compared with CTE, MRE provides better soft-tissue contrast that can reliably differentiate between inflammation and chronic fibrotic changes. T2-weighted signal increase is associated with inflammation and edema and is a marker of active Crohn’s disease.16 However, in CTE, active Crohn’s disease may look similar to chronic fibrotic changes. Single-shot T2W imaging combined with fat suppression employing the SPAIR technique provides optimal sensitivity and specificity for active Crohn’s disease.15,16,23 Earlier studies either did not use fat-suppressed T2 or did not use optimized fat suppression and may not have appreciated the full utility of MRE.4,36,37 Other forms of fat suppression, such as simple inversion-recovery or chemical shift spoiling, typically demonstrate higher noise, less uniform fat suppression, and increased through-plane motion sensitivity to bowel peristalsis.23 Although MRE is relatively insensitive to early disease, or disease confined to the mucosa, it is effective at evaluating the severity and extent of submucosal pathology and extra-intestinal complications.38 T2W imaging with fat saturation has shown high accuracy for measuring inflammation and acute disease activity compared to endoscopy, biopsy, and CT. A comprehensive examination of inflammatory bowel pathology can be provided with a combination of MRE and endoscopic techniques. Additionally, MRE is further improved with additional refinements in fat suppression such as multi-echo Dixon 3D GRE, DWI, and perfusion techniques. We predict eventual integration of MRE into routine CD activity scoring for longitudinal monitoring and management of therapeutic interventions.
References
- Burisch J, Munkholm P. Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol. 2013;29(4):357-362.
- Mekhjian HS, Switz DM, Melnyk CS, et al. Clinical features and natural history of Crohn’s disease. Gastroenterology 1979;77(4 pt.2):898-906.
- Kopylov U, Yung D, Vijayan S. Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel Crohn’s disease: Systemic review and meta-analysis. Dig Liver Dis. 2017;49(8):854-863.
- Albert JG, Martiny F, Krummenerl A, et al. Diagnosis of small bowel Crohn’s disease: A prospective comparison of capsule endoscopy with magnetic resonance imaging and fluoroscopic enteroclysis. Gut. 2005;54:1721–1727.
- Golder SK, Schreyer AG, Endlicher E, et al. Comparison of capsule endoscopy and magnetic resonance (MR) enteroclysis in suspected small bowel disease. Int J Colorectal Dis. 2006;21(2):97–104.
- Tillack C, Seiderer J, Brand S, et al. Correlation of magnetic resonance enteroclysis (MRE) and wireless capsule endoscopy (CE) in the diagnosis of small bowel lesions in Crohn’s disease. Inflamm Bowel Dis. 2008;14(9):1219–1228.
- Lee SS, Kim AY, Yang SK, et al. Crohn disease of the small bowel: Comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251(3):751–761.
- Nylund K, Hausken T, Gilja O. Ultrasound and inflammatory bowel disease. Ultrasound. 2010;26(1):3-15.
- Panes J, Bouhnik Y, Reinisch W et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556-585.
- Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. AJR Am J Roentgenol. 2009;193(1):113-121.
- Schmidt S, Lepori D, Meuwly JY, et al. Prospective comparison of MR enteroclysis with multidetector spiral-CT enteroclysis: Interobserver agreement and sensitivity by means of ‘sign-by-sign’ correlation. Eur Radiol. 2003;13(6):1303-1311.
- Low RN, Francis IR, Politoske D, Bennett M. Crohn’s disease evaluation: Comparison of contrastenhanced MR imaging and single-phase helical CT scanning. J Magn Reson Imaging. 2000;11(2):127-135.
- Gale H, Sharatz S, Taphey M. Comparison of CT enterography and MR enterography imaging features of active Crohn disease in children and adolescents. Pediatr Radiol. 2017;47(10):1321-1328.
- Jaffe TA, Gaca AM, Delaney S, et al. Radiation doses from small-bowel follow-through and abdominopelvic MDCT in Crohn’s disease. AJR Am J Roentgenol. 2007;189(5):1015–1022.
- Martin DR, Lauenstein T, Sitaraman SV. Utility of magnetic resonance imaging in small bowel Crohn’s disease. Gastroenterology. 2007; 133(2):385–390.
- Udayasankar UK, Martin D, Lauenstein T, et al. Role of spectral presaturation attenuated inversionrecovery fat-suppressed T2-weighted MR imaging in active inflammatory bowel disease. J Magn Reson Imaging. 2008;28(5):1133-1140.
- Martin DR, Danrad R, Herrmann K, et al. Magnetic resonance imaging of the gastrointestinal tract. Top Magn Reson Imaging. 2005;16(1):77-98.
- Punwani S, Rodriguez-Justo M, Bainbridge A, et al. Mural inflammation in Crohn disease: Locationmatched histologic validation of MR imaging features. Radiology. 2009;252(3):712-720.
- Fornasa F, Benassuti C, Benazzato L. Role of magnetic resonance enterography in differentiating between fibrotic and active inflammatory small bowel stenosis in patients with Crohn’s disease. J Clin Imaging Sci. 2011(1);1:35.
- Ha CY, Kumar N, Raptis CA, et al. Magnetic resonance enterography: Safe and effective imaging for stricturing Crohn’s disease. Dig Dis Sci. 2011; 56(10):2906–2913.
- Ajaj W, Lauenstein TC, Langhorst J, et al. Small bowel hydro-MR imaging for optimized ileocecal distension in Crohn’s disease: Should an additional rectal enema filling be performed? J Magn Reson Imaging. 2005; 22(1):92-100.
- Narin B, Ajaj W, Gohde S, et al. Combined small and large bowel MR imaging in patients with Crohn’s disease: A feasibility study. Eur Radiol. 2004;14(9):1535-1542.
- Lauenstein TC, Sharma P, Hughes T, et al. Evaluation of optimized inversion-recovery fat-suppression techniques for T2-weighted abdominal MR imaging. J Magn Reson Imaging. 2008;27(6):1448-1454.
- Florie J, Wasser MN, Arts-Cieslik K, et al. Dynamic contrast-enhanced MRI of the bowel wall for assessment of disease activity in Crohn’s disease. AJR Am J Roentgenol. 2006;186(5):1384–1392.
- Maccioni F, Bruni A, Viscido A, et al. MR imaging in patients with Crohn disease: Value of T2- versus T1-weighted gadolinium-enhanced MR sequences with use of an oral superparamagnetic contrast agent. Radiology. 2006;238(2):517–530.
- Oto A, Kayhan A, Williams JT, et al. Active Crohn’s disease in the small bowel: Evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging. 2011;33(3):615-624.
- Neubauer H, Pabst T, Dick A, et al. Small-bowel MRI in children and young adults with Crohn disease: Retrospective head-to-head comparison of contrast-enhanced and diffusion-weighted MRI. Pediatr Radiol. 2013; 43(1):103-114.
- Dubron C, Avni F, Boutry N. Prospective evaluation of free-breathing diffusion-weighted imaging for the detection of inflammatory bowel disease with MR enterography in childhood population. Br J Radiol. 2016;89(1060):20150840. doi: 10.1259/bjr.20150840. Epub 2016 Feb 3.
- Tolan DJ, Greenhalgh R, Zealley IA, Halligan S, Taylor SA. MR enterographic manifestations of small bowel Crohn disease. Radiographics. 2010; 30(2):367-384.
- Rimola J, Rodriguez S, Garcia-Bosch O et al. Role of 3.0-T MR colonography in the evaluation of inflammatory bowel disease. Radiographics 2009;29(3):701–19.
- Ephgrave K. Extra-intestinal manifestations of Crohn’s disease. Surg Clin North Am. 2007:87(3):673-80.
- Jiang X, Asbach P, Hamm B, Xu K, Banzer J. MR imaging of distal ileal and colorectal chronic inflammatory bowel disease — diagnostic accuracy of 1.5T and 3T MRI compared to colonoscopy. Int J Colorectal Dis. 2014;29(12):1541–1550.
- Liu W, Liu J, Xiao W. A diagnostic accuracy meta-analysis of CT and MRI for the evaluation of small bowel Crohn’s disease. Acad Radiol. 2017;24(10):1216-1225.
- Campari A, Napolitano M, Zuin G. Colonic Inflammation in pediatric inflammatory bowel disease: detection with magnetic resonance enterography. Pediatr Radiol. 2017;47(7):850-859.
- Ordás I, Rimola J, García-Bosch O, et al. Diagnostic accuracy of magnetic resonance colonography for the evaluation of disease activity and severity in ulcerative colitis: a prospective study. Gut. 2013;62(11):1566-1572.
- Shoenut JP, Semelka RC, Magro CM, et al. Comparison of magnetic resonance imaging and endoscopy in distinguishing the type and severity of inflammatory bowel disease. J Clin Gastroenterol. 1994;19(1):31–35.
- Sempere GA, Martinez Sanjuan V, Medina Chulia E, et al. MRI evaluation of inflammatory activity in Crohn’s disease. AJR Am J Roentgenol. 2005; 184(6):1829–1835.
- Lawrance IC, Welman CJ, Shipman P, et al. Small bowel MRI enteroclysis or follow through: Which is optimal? World J Gastroenterol. 2009;15(42):5300–5306.
Citation
H A, P T, BT K, DR M. Magnetic resonance enterography in inflammatory bowel disease. Appl Radiol. 2019;(1):9-15.
February 6, 2019