Top Ten clinical indications for coronary CT angiography





Dr. Achenbach is a Professor of Medicine, Department of Cardiology, University of Erlangen, Erlangen, Germany.
Imaging of the coronary arteries requires high temporal and spatial resolution. Invasive, catheter-based coronary angiography is the clinical standard tool for assessment of the coronary arteries, but it has several shortcomings: First of all, it is an invasive procedure and, as such, is associated with a certain morbidity and mortality, which in most cases is a consequence of the required arterial access. 1 Secondly, some limitations are due to its projectional nature. For example, exact delineation of the 3-dimensional (3D) anatomy can be problematic, which may cause difficulties, eg, in the context of coronary anomalies. Finally, cardiac catheterization requires elaborate equipment that is not available at every hospital or outpatient setting, dedicated and well-trained staff is necessary, and associated costs are high.
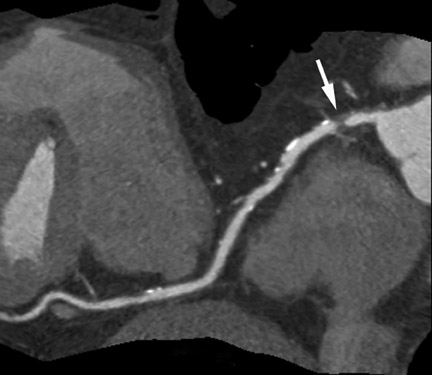
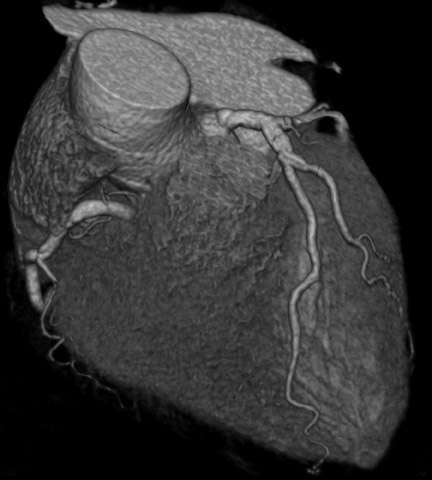
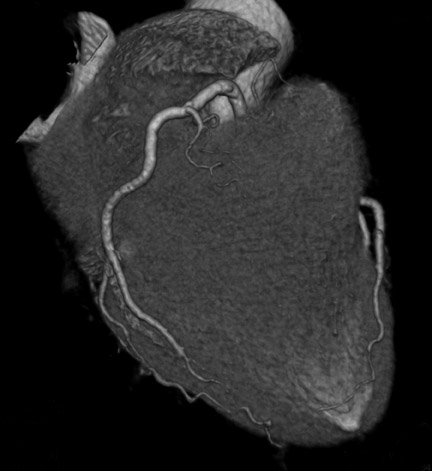
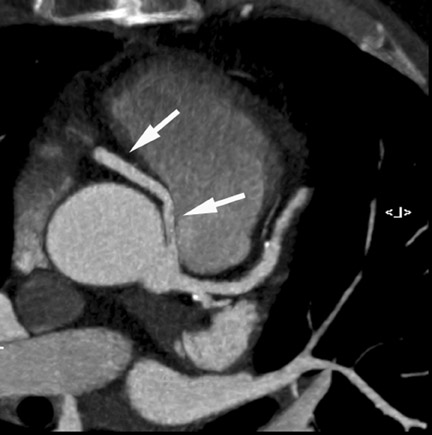
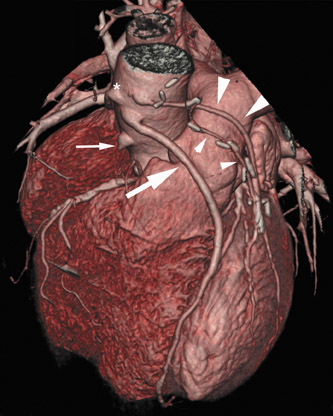
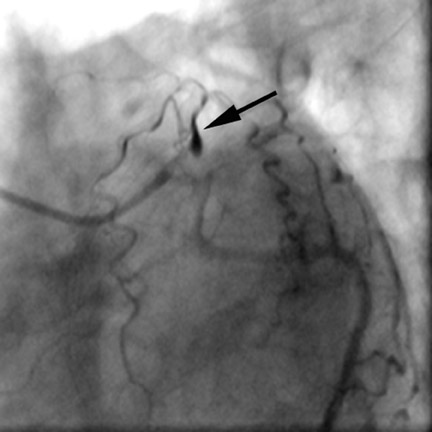
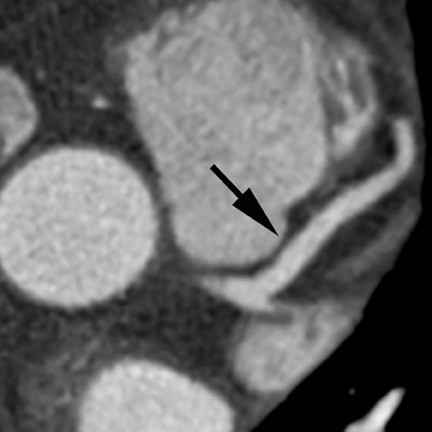
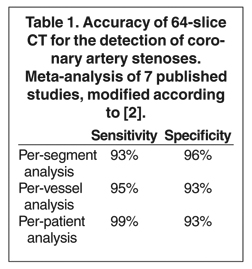
Computed tomographic (CT) technology has progressed rapidly over the past several years. Both spatial and temporal resolution have steadly been improved, and the introduction of 64-slice CT has made coronary CT angiography (CTA) a relatively robust and stable tool for coronary artery visualization (Figure 1). A recent meta-analysis showed high accuracies for the detection of coronary artery stenoses (CAS) by 64slice CT 2 (Table 1). Of course, there are limitations as compared with the invasive angiogram: Limited temporal resolution can reduce image quality, especially if heart rates are above 60 beats per minute (bpm). 3-6 This limitation may not be as pronounced for the newer dual-source CT scanners. 7-9 Since data acquired over several heartbeats are necessary to acquire a complete data set, coronary CTA is not reliably possible in patients with arrhythmias (scanner design concepts with ≥256 slices may help overcome this limitation). For all scanner generations, the spatial resolution is lower than that of invasive angiography, making analysis of smaller side branches and distal vessel segments impossible--in most studies, analysis was limited to segments of ≥1.5 mm in diameter. Also, there is a tendency to overestimate the degree of stenosis in CT as compared with the invasive angiogram, and extensive calcifications can render image interpretation impossible. Finally, CTA is limited to diagnosis. In patients with a high pretest likelihood of disease, performing an invasive, catheter-based coronary angiogram will often be much more appropriate because it offers the option of immediate treatment.
While invasive angiography will remain the clinical gold standard for coronary artery visualization for the foreseeable future, CT imaging has some potential advantages over invasive angiography. In addition to being noninvasive, its tomographic nature allows for easier and unambiguous identification of the 3D anatomy of the coronary vessels, which can be useful in cases of coronary anomalies. Furthermore, its cross-sectional nature permits visualization not only of the contrast-enhanced coronary artery lumen, but also of the vessel wall (if image quality is adequate). In this way, atherosclerotic plaque can become visible, which is undetectable in the invasive coronary angiogram (Figure 1).
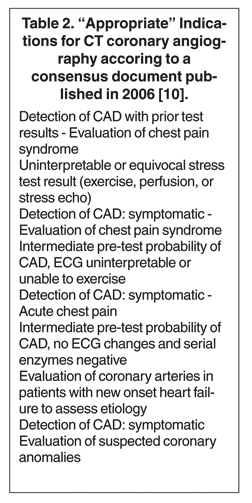
For potential clinical applications, the advantages and diasdvantages of CTA must be weighed against those of invasive coronary angiography. In a consensus document, a group of experts from various professional societies have listed "appropriate" clinical indications for coronary CTA, based mostly on the considerations outlined above (Table 2). 10 Several additional, more infrequently used indications are conceivable. Ultimately, the individual clinical situation will help make the decision for or against coronary CTA; and many issues--such as the patient's heart rate, body weight, or ability to perform a breath-hold, as well as contraindications to contrast or problems with vascular access (which may make invasive angiography more prone to complication)--will play a role in the decision-making process. In the following section, potential clinical indications for the use of coronary CTA are outlined as a "Top Ten" list, from the clearest to the least robust and frequent indications. By necessity, this list is a subjective interpretation of the author and is likely to undergo modifications as technology progresses.
1. Ruling out significant luminal stenoses in stable patients with suspected coronary stenoses, but intermediate pretest likelihood of disease
The available literature convincingly demonstrates that coronary CTA, if expertly performed, has a high negative predictive value and thus allows one to rather reliably rule out the presence of CAS. 2,11,12 The aim is to avoid an otherwise necessary invasive coronary angiogram if CT shows the absence of clinically relevant CAS. Based on clinical considerations, but also for statistical reasons, CT imaging will be most useful in patients with an intermediate likelihood of CAS. In patients with a very low pretest likelihood, the false-positive rate may be too high, and in patients with a very high pretest likelihood, sensitivity may not be sufficiently high. Meijboom et al 13 have recently presented a careful analysis of the diagnostic value of coronary CTA, stratified according to the pretest likelihood of disease. They also found that the technique is most useful in patients with a low-to-intermediate likelihood of CAS.
This is certainly the most prominent and frequent clinical indication of cardiac CT and can be beneficially applied, for example, in patients with rather atypical symptoms, patients with unclear stress test results, or patients in whom the stress test result contradicts the clinical assessment (Figure 2). Similarly, coronary CTA has been shown to rule out CAS in patients with left bundle branch block of unknown etiology 6 or in patients with new onset heart failure. 14 The goal of performing CTA in this context is always to avoid invasive angiography if the CT scan shows good evaluability and an absence of CAS.
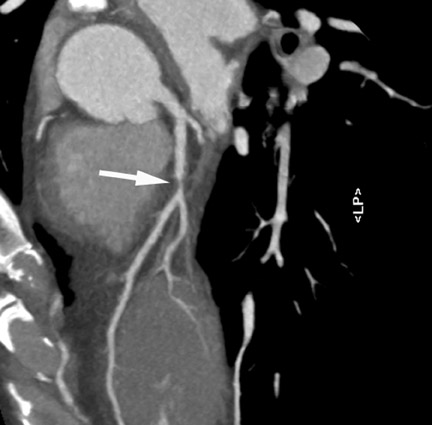
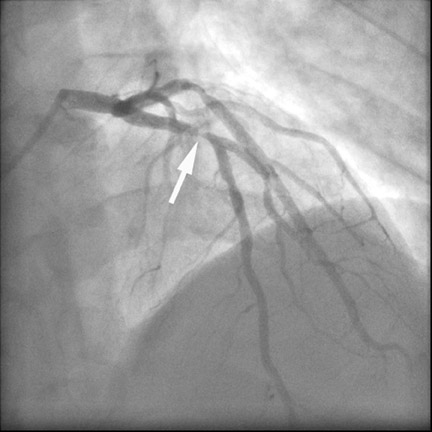
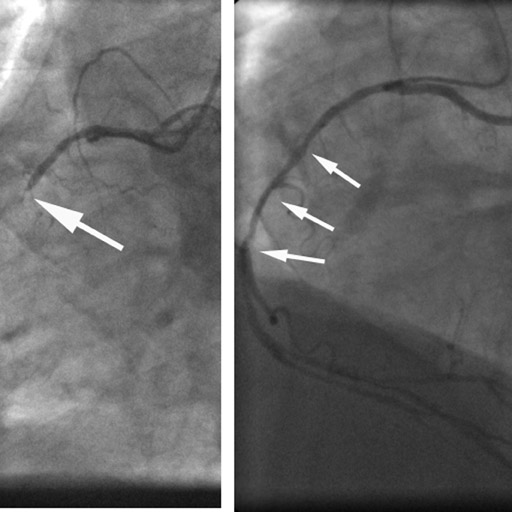
2. Ruling out coronary artery disease in acute chest pain
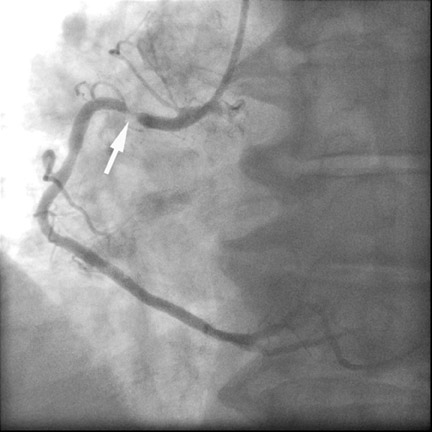
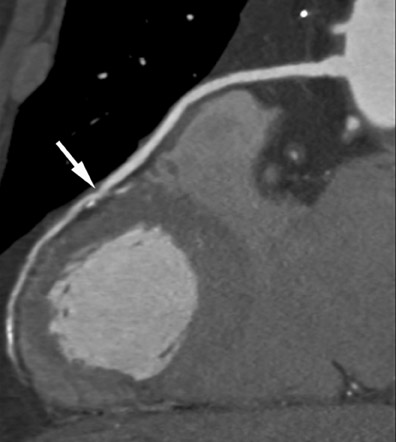
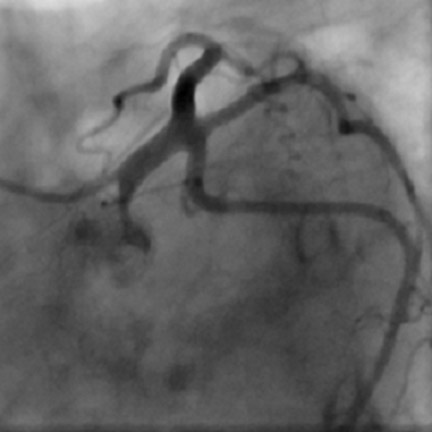
Especially if the electrocardiogram and myocardial enzymes are normal, many patients who present to the emergency room with acute chest pain have a relatively low likelihood of coronary artery disease. Further testing is often necessary to rule in or rule out the presence of coronary artery disease. In these patients, coronary CTA can be a useful tool to rapidly assess the coronary arteries for the presence of coronary lesions (Figure 3). Some initial studies have shown the high accuracy of CT to identify patients who have CAS in the setting of acute chest pain, 15,16 as well as cost-effectiveness in comparison with standard diagnostic algorithms, 17 and a favorable long-term outcome of patients who were discharged based on a coronary CT examination that showed the absence of stenosis. 18 It can be expected that the use of CT in patients presenting with acute or unstable chest pain will be one of the most frequent indications for coronary CTA.
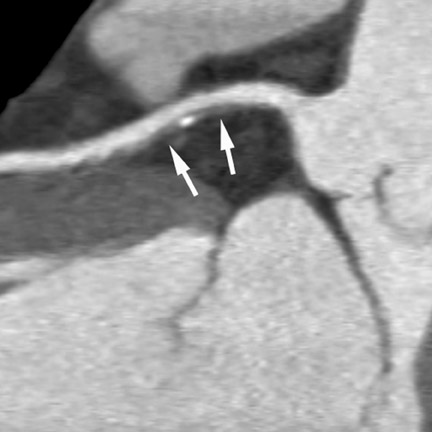
3. Coronary anomalies
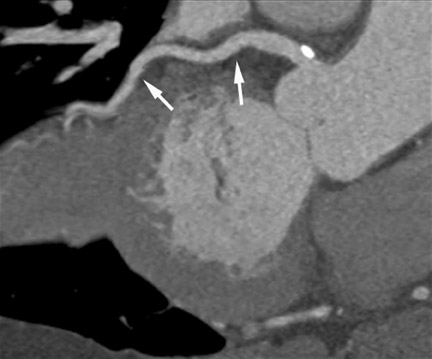
Multidetector CT (MDCT) can classify both the origin and also the often complex course of anomalous coronary vessels 19-22 (Figure 4). Magnetic resonance coronary angiography may be an alternative in experienced hands, and the necessity for contrast agent injection and radiation exposure are certainly drawbacks of CT imaging. Coronary CTA is the method of choice for the work-up of known or suspected anomalous coronary vessels because of the ease of data acquisition and the predictability with which a high-resolution data set with optimal image quality for evaluation can be expected. The use of CTA in the setting of coronary anomalies has been classified as a clinically "appropriate" indication. 10
4. Ruling out stenoses before noncoronary cardiac surgery
Cardiothoracic surgeons often require invasive coronary angiography to rule out CAS in patients who are scheduled for cardiac surgery for noncoronary reasons (eg, valve replacement or resection of tumors) or for surgery of the ascending aorta. Stress testing is not reliable enough, and symptoms may be masked by the underlying disease. If these patients do not have arrhythmias (atrial fibrillation may, in fact, be quite common in patients with mitral valve disease) and if they are clinically sufficiently stable, CTA may be a useful tool to clear them for cardiac surgery without having to perfom invasive angiography. One study has specifically addressed the use of 64-slice CT to detect CAS in patients prior to aortic valve replacement. 23 While 35 of 105 patients could not be studied by CT because of atrial fibrillation or renal failure, CT successfully detected all 18 patients with high-grade CAS in the remaining 50 individuals (sensitivity 100%, specificity 82%). It may thus be assumed that coronary CTA will be useful in certain subgroups of patients before valvular or other noncoronary cardiac surgery, although not all patients will be candidates for CT scanning.
5. Determine patency of coronary artery bypass grafts
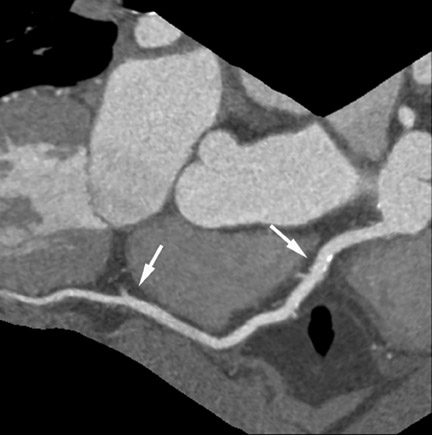
CT angiography has a high accuracy for the detection of bypass graft stenosis and occlusion. 24-31 Bypass grafts have a larger caliber than native coronary arteries, and they are subjected to motion to a lesser extent than native coronary vessels, which favorably influences image quality (Figure 5). However, the coronary arteries themselves can be very difficult to assess by CT in patients after bypass surgery: they often have severe atherosclerosis, including pronounced calcification, and frequently are of small caliber, which makes their evaluation challenging. 29,30 The most recent scanner generations have higher temporal and spatial resolution and may thus allow more reliable assessment of the coronary system in patients with bypass grafts. A recent study performed by 64-slice CT found a sensitivity and specificity of only 86% and 76% for the detection of stenoses in the native coronary arteries of patients with bypass surgery. 30 Therefore, in clinical cases that require only the assessment of bypass grafts (eg, if not all grafts were found during invasive angiography or in the early postoperative situation), the use of CTA may be beneficial. If, however, the clinical situation requires assessment of both the bypass grafts and native coronary artery system, the value of CTA is limited.
6. Using CT as an alternative when cardiac catheterization is impossible or carries a high risk
In some patients, assessment of the coronary arteries may be necessary, but invasive angiography may be associated with an increased risk- eg, in patients with bleeding disorders, in patients with dissection of the ascending aorta, or in patients with large endocarditic vegetations on the aortic valve. Even though this does not constitute a frequent clinical situation, coronary CTA may be useful and beneficial in these instances. The use of CTA may be extended beyond low-to-intermediate-risk patients if such factors are present that would constitute a particularly high risk for invasive angiography.
7. Clarifying unclear findings after invasive angiography
Infrequently, coronary anatomy and pathology may not be entirely clear even after an invasive angiogram. Most frequently, this will be in the context of coronary anomalies, as described above, but some other situations exist in which CTA may be useful even after an invasive angiogram. Very infrequently, for example, a CT scan may be helpful if an invasive angiogram fails to fully clarify the presence of coronary stenosis at the right or left coronary ostium. Another potential situation is when a completely obstructed side branch is suspected, but not clearly visualized in the invasive angiogram. In such cases and in some other situations, CT can often clarify the clinical question (Figure 6).
8. Providing peri-interventional information for percutaneous coronary intervention
CT can provide information that could be useful in the context of percutaneous coronary intervention. One study has shown that in cases of chronic total coronary artery occlusion, CT can more reliably identify parameters that will predict the success of interventional revascularization than the invasive angiogram can. The most important parameters are the length and the extent of calcification of the occluded segment 32 (Figure 7). Similarly, CT can provide more exact information about plaque distribution and bifurcation angles than the invasive angiogram can, 33 which may be helpful in choosing the best strategy for stenting of bifurcation lesions.
9. Assessing coronary artery stents
The visualization of the lumen within coronary artery stents by MDCT is possible. However, artifacts caused by the stent material may create problems in a substantial number of patients, especially in combination with calcium or motion (Figure 8). The ability to assess stents concerning restenosis depends on many factors, including stent type 34 and stent diameter as well as image noise, which in turn is heavily influenced by body weight. While some more recent studies suggest that the analysis of large stents (with a diameter of ≥3.5 mm) may be possible by CT, 35-44 the available data is very limited. Because of the relatively high number of unevaluable studies and the somewhat limited positive predictive value, stent imaging should currently not be considered a routine application for coronary CTA. The exceptional application would be limited to patients with stents of a relatively large diameter in proximal vessel segments, in whom invasive angiography cannot be performed without an increased risk of complications.
10. Determining the presence and extent of coronary atherosclerotic plaque
Besides detecting CAS, CTA is also able to reveal the presence of nonstenotic coronary atherosclerotic plaque (Figure 9). 45-49 In addition, CT may potentially determine parameters that are connected to plaque "vulnerability," such as the extent of remodeling, 50 the CT attenuation within the plaque (with lower CT attenuation assumed to be corresponding to higher plaque "vulnerability"), 48,49,51-53 the degree of stenosis, and plaque dimensions. 48,54 This has led to the concept of using contrast-enhanced CT for risk stratification in order to identify asymptomatic individuals with an elevated risk for future myocardial infarction. However, there currently is very little clinical data to support such applications of cardiac CT. Several smaller studies have retrospectively analyzed plaque characteristics by CT in patients after acute coronary syndromes compared with patients with stable angina and have found a higher percentage of noncalcified plaque and more positive remodeling in patients and lesions responsible for cardiac events. 55-57 However, it is problematic that CT data acquisition in these patients was performed after the ischemic event, and plaque rupture may have contributed to morphologic changes of the atherosclerotic lesion as seen in CT. Only one prospective trial is currently available. Pundziute et al 58 followed 100 patients who underwent coronary CTA for a mean period of 16 months and reported that patients with nonobstructive plaque detected by MDCT had a higher cardiovascular event rate than individuals without any plaque (most of these events, however, may have been revascularizations).The available data provide some indication that assessment of noncalcified plaque by coronary CTA may have predictive value in asymptomatic individuals. However, plaque imaging by CT requires the injection of a contrast agent and also involves significant radiation exposure; both these factors should be considered, and CT should be used as a clinical tool only if very strong superiority over other methods of risk prediction has clearly been shown. This is currently not the case, and contrast-enhanced CT for plaque visualization should be restricted to research settings.
Conclusion
Coronary CTA has numerous clinical applications. Its most prominent role is in the assessment of patients with possible coronary artery stenoses, but a relatively low likelihood of disease, with the aim to rule out coronary stenoses and avoid the need for an invasive coronary angiogram. This includes patients with various clinical scenarios, such as atypical symptoms, unclear electrocardiographic changes or stress test results, patients with new onset of heart failure, and patients before noncoronary cardiac surgery. Assessment of coronary anomalies is another strong indication, but much less frequent. Other applications of CT are possible but are not currently backed by sufficient amounts of data such as to provide peri-interventional information, to detect in-stent restenosis, or to provide risk stratification. Eventually, all large-scale applications of coronary CTA will need to be backed by prospective clinical trials that provide evidence for the clincal benefit of using CT in the respective situation. In fact, reimbursement for cardiac CT may hinge on that data. It can be expected that suitable trials will be performed in the coming years and that the progress in CT technology will lead to a further expansion of the range of possible clinical applications.
REFERENCES
- Kennedy JW. Complications associated with cardiac catheterization and angiography. Cath Cardiovasc Diagn. 1982;8:5-11.
- Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, et al. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: Meta-analysis. Radiology. 2007;44:419-428.
- Leschka S, Wildermuth S, Boehm T, et al. Noninvasive coronary angiography with 64-section CT: Effect of average heart rate and heart rate variability on image quality. Radiology. 2006;241:378-385.
- Hoffmann MH, Shi H, Manzke R, et al. Noninvasive coronary angiography with 16-detector row CT: Effect of heart rate. Radiology. 2005;234:86-97.
- Herzog C, Arning-Erb M, Zangos S, et al. Multi-detector row CT coronary angiography: Influence of reconstruction technique and heart rate on image quality. Radiology. 2006;238:75-86
- Ghostine S, Caussin C, Daoud B, et al. Non-invasive detection of coronary artery disease in patients with left bundle branch block using 64-slice computed tomography. J Am Coll Cardiol. 2006;48:1929-1934.
- Leber AW, Johnson T, Becker A, et al. Diagnostic accuracy of dual-source multi-slice CT-coronary angiography in patients with an intermediate pretest likelihood for coronary artery disease. Eur Heart J. 2007;28:2354-2360.
- Scheffel H, Alkadhi H, Plass A, et al. Accuracy of dual-source CT coronary angiography: First experience in a high pre-test probability population without heart rate control. Eur Radiol. 2006;16:2739-2747.
- Ropers U, Ropers D, Pflederer T, et al. Influence of heart rate on the diagnostic accuracy of dual-source computed tomography coronary angiography. J Am Coll Cardiol. 2007;50:2393-2398.
- Hendel RC, Patel MR, Kramer CM, et al. ACCF/ ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. J Am Coll Cardiol. 2006;48:1475-1497.
- Hamon M, Biondi-Zoccai GG, Malagutti P, et al. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: A meta-analysis. J Am Coll Cardiol. 2006;48:1896-1910.
- Shroeder S, Achenbach S, Bengel F, et al. Cardiac computed tomography: Indications, applications, limitations, and training requirements: Report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J. 2008;In press.
- Meijboom WB, van Mieghem CA, Mollet NR, et al. 64-slice computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coronary artery disease. J Am Coll Cardiol. 2007;50:1469-1475.
- Andreini D, Pontone G, Pepi M, et al. Diagnostic accuracy of multidetector computed coronary tomography angiography in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2007;49:2044-2050.
- Hoffmann U, Nagurney JT, Moselewski F, et al. Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circulation. 2006;114:2251-2260. Erratum in: Circulation. 2006;114:e651.
- Meijboom WB, Mollet NR, Van Mieghem CA, et al. 64-slice computed tomography coronary angiography in patients with non-ST elevation acute coronary syndrome. Heart. 2007;93:1386-1392.
- Goldstein JA, Gallagher MJ, O'Neill WW, et al. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49:863-871.
- Rubinshtein R, Halon DA, Gaspar T, et al. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation. 2007;115:1762-1768.
- Hollander JE, Litt HI, Chase M, et al. Computed tomography coronary angiography for rapid disposition of low-risk emergency department patients with chest pain syndromes. Acad Emerg Med. 2007;14:112-116.
- Deibler AR, Kuzo RS, Vöhringer M. Imaging of congenital coronary anomalies with multislice computed tomography. Mayo Clin Proc. 2004;79: 1017-1023.
- Datta J, White CS, Gilkeson RC, et al. Anomalous coronary arteries in adults: Depiction at multi-detector row CT angiography. Radiology. 2005;235:812-818.
- Dodd JD, Ferencik M, Liberthson RR, et al. Congenital anomalies of coronary artery origin in adults: 64-MDCT appearance. AJR Am J Roentgenol. 2007; 188:W138-W146.
- Meijboom WB, Mollet NR, Van Mieghem CA, et al. Pre-operative computed tomography coronary angiography to detect significant coronary artery disease in patients referred for cardiac valve surgery. J Am Coll Cardiol. 2006;48:1658-1665.
- Nieman K, Pattynama PM, Rensing BJ, et al. Evaluation of patients after coronary artery bypass surgery: CT angiographic assessment of grafts and coronary arteries. Radiology. 2003;229:749-756.
- Martuscelli E, Romagnoli A, D'Eliseo A, et al. Evaluation of venous and arterial conduit patency by 16-slice spiral computed tomography. Circulation. 2004;110: 3234-3238.
- Schlosser T, Konorza T, Hunold P, et al. Noninvasive visualization of coronary artery bypass grafts using 16-detector row computed tomography. J Am Coll Cardiol. 2004;44:1224-1229.
- Chiurlia E, Menozzi M, Ratti C, et al. Follow-up of coronary artery bypass graft patency by multislice computed tomography. Am J Cardiol. 2005;95:1094-1097.
- Feuchtner GM, Schachner T, Bonatti J, et al. Diagnostic performance of 64-slice computed tomography in evaluation of coronary artery bypass grafts. AJR Am J Roentgenol. 2007;189:574-580.
- Salm LP, Bax JJ, Jukema JW, et al. Comprehensive assessment of patients after coronary artery bypass grafting by 16-detector-row computed tomography. Am Heart J. 2005;150:775-781.
- Ropers D, Pohle FK, Kuettner A, et al. Diagnostic accuracy of noninvasive coronary angiography in patients after bypass surgery using 64-slice spiral computed tomography with 330-ms gantry rotation. Circulation. 2006;114:2334-2341.
- Meyer TS, Martinoff S, Hadamitzky M, et al. Improved noninvasive assessment of coronary artery bypass grafts with 64-slice computed tomographic angiography in an unselected patient population. J Am Coll Cardiol. 2007;49:946-950.
- Mollet NR, Hoye A, Lemos PA, et al. Value of preprocedure multislice computed tomographic coronary angiography to predict the outcome of percutaneous recanalization of chronic total occlusions. Am J Cardiol. 2005;95:240-243.
- Pflederer T, Ludwig J, Ropers D, et al. Measurement of coronary artery bifurcation angles by multidetector computed tomography. Invest Radiol. 2006;41:793-798.
- Maintz D, Seifarth H, Raupach R, et al. 64-slice multidetector coronary CT angiography: In vitro evaluation of 68 different stents. Eur Radiol. 2006;16:818-826.
- Gilard M, Cornily JC, Pennec PY, et al. Assessment of coronary artery stents by 16 slice computed tomography. Heart. 2006;92:58-61.
- Schuijf JD, Bax JJ, Jukema JW, et al. Feasibility of assessment of coronary stent patency using 16-slice computed tomography. Am J Cardiol. 2004;94:427-430.
- Gaspar T, Halon DA, Lewis BS, et al. Diagnosis of coronary in-stent restenosis with multidetector row spiral computed tomography. J Am Coll Cardiol. 2005;46:1573-1579.
- Gilard M, Cornily JC, Rioufol G, et al. Noninvasive assessment of left main coronary stent patency with 16-slice computed tomography. Am J Cardiol. 2005;95:110-112.
- Van Mieghem CA, Cademartiri F, Mollet NR, et al. Multislice spiral computed tomography for the evaluation of stent patency after left main coronary artery stenting: A comparison with conventional coronary angiography and intravascular ultrasound. Circulation. 2006;114:645-653.
- Rixe J, Achenbach S, Ropers D, et al. Assessment of coronary artery stent restenosis by 64-slice multi-detector computed tomography. Eur Heart J. 2006; 27:2567-2572.
- Oncel D, Oncel G, Karaca M. Coronary stent patency and in-stent restenosis: Determination with 64-section multidetector CT coronary angiography- initial experience. Radiology. 2007; 242:403-409.
- Ehara M, Kawai M, Surmely JF, et al. Diagnostic accuracy of coronary in-stent restsnosis using 64-slice computed tomography.J Am Coll Cardiol.2007; 49:951-959.
- Rist C, von Ziegler F, Nikolaou K, et al. Assessment of coronary artery stent patency and restenosis using 64-slice computed tomography. Acad Radiol.2006;13:1465-1473.
- Cademartiri F, Schuijf JD, Pugliese F, et al. Usefulness of 64-slice multislice computed tomography coronary angiography to assess in-stent restenosis. J Am Coll Cardiol. 2007;49:2204-2210.
- Becker CR, Knez A, Ohnesorge B, et al. Imaging of noncalcified coronary plaques using helical CT with retrospective ECG gating. AJR Am J Roentgenol. 2000;175:423-424.
- Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: A segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14-17.
- Leber AW, Knez A, Becker A, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: A comparative study with intracoronary ultrasound. J Am Coll Cardiol. 2004;43:1241-1247.
- Leber AW, Becker A, Knez A, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: A comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006;47:672-627.
- Schroeder S, Kopp AF, Baumbach A, et al. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J Am Coll Cardiol. 2001;37:1430-1435.
- Achenbach S, Ropers D, Hoffmann U, et al. Assessment of coronary remodeling in stenotic and nonstenotic coronary atherosclerotic lesions by multidetector spiral computed tomography. J Am Coll Cardiol. 2004;43:842-847.
- Caussin C, Ohanessian A, Ghostine S, et al. Characterization of vulnerable nonstenotic plaque with 16-slice computed tomography compared with intravascular ultrasound. Am J Cardiol. 2004;94:99-100.
- Carrascosa PM, Capuñay CM, Garcia-Merletti P, et al. Characterization of coronary atherosclerotic plaques by multidetector computed tomography. Am J Cardiol. 2006;97:598-602.
- Pohle K, Achenbach S, Macneill B, et al. Characterization of non-calcified coronary atherosclerotic plaque by multi-detector row CT: Comparison to IVUS. Atherosclerosis. 2007;190:174-180.
- Moselewski F, Ropers D, Pohle K, et al. Comparison of measurement of cross-sectional coronary atherosclerotic plaque and vessel areas by 16-slice multi-detector computed tomography versus intra-vascular ultrasound. Am J Cardiol. 2004;94: 1294-1297.
- Hoffmann U, Moselewski F, Nieman K, et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47:1655-1662.
- Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319-326.
- Schuijf JD, Beck T, Burgstahler C, et al. Differences in plaque composition and distribution in stable coronary artery disease versus acute coronary syndromes; non-invasive evaluation with multi-slice computed tomography. Acute Card Care. 2007;9: 48-53.
- Pundziute G, Schuijf JD, Jukema JW, et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007; 49:62-70.
