The role of CT angiography in aortic stent-grafting
Images

Dr. Foley is a Professor of Radiology, Medical College of Wisconsin, Milwaukee, WI.
Aortic aneurysm occurs in 3% of people over the age of 50, and 5% to 8% of men over the age of 65. Men who smoke and people with hypertension are at highest risk. If left untreated, an aortic aneurysm with a diameter exceeding 6 cm has a 5-year mortality rate of 25% to 40%. 1,2
Endovascular stent-grafting is an increasingly common treatment for aortic aneurysm. Early on, digital subtraction angiography and intravascular ultrasound were used intraoperatively to take critical measurements of aortic length and diameter, thus guiding selection and placement of the aortic stent-graft. Today, CT angiography (CTA) can be used prior to the stent-grafting to create an anatomic model of the aorta. Quantitative measurements enable preprocedural selection of the stent-graft and planning for its placement. CT angiography also evaluates patient characteristics that infiuence procedural success, including the viability of iliac access, and the presence and quantity of calcification and atheromatous debris in the superior neck.
This article will discuss screening for and surveillance of aortic aneurysms; the role of CTA in patient selection and planning for endograft therapy; the performance and interpretation of CTA, including the use of quantitative analysis tools; and the recognition and management of stent-graft complications.
Patient evaluation
Aortic aneurysm can be defined in various ways. A practical definition is an abdominal aorta with an external diameter >4 cm (in the case of a fusiform aneurysm); or a focal dilatation >3 cm in conjunction with a proximal aortic luminal diameter <1.5 cm. 3
Ultrasound is the preferred technique for screening patients for aortic aneurysm. It is recommended that patients who have an abdominal aortic aneurysm ≥4 cm in diameter undergo surveillance sonography at intervals of 6 to 12 months. Once the diameter reaches approximately 5.5 cm, an intervention should be considered, balancing the risks of treatment against those of leaving the aneurysm untreated. Another indication for intervention is an abdominal aortic aneurysm that increases by >5 mm in diameter in a 6-month interval. Due to the inherent risks of therapy and reduced life expectancy, a larger aortic diameter may be required in an older patient be-fore recommending intervention.
An aortic aneurysm can be repaired surgically or with endovascular stenting. Endovascular stenting is becoming more common because it avoids many of the disadvantages of surgery, including the need for laparotomy. Laparotomy involves aortic cross-clamping, which, in the case of juxtarenal or suprarenal aneurysms, can result in ischemia of the intestine and the kidneys; surgical opening of an aneurysmal sac causes obligatory blood loss; and the surgeon may inadvertently lacerate major vessels, such as a retroaortic left renal vein.
Several critical anatomic issues must be evaluated before endovascular stent-grafting. These include the availability of iliac access, which is necessary for introduction of the stent-graft device; the length and diameter of the proximal placement zone, between the renal vascular pedicle and the upper margin of the aneurysm; the degree of atheromatous burden in the superior neck of the aneurysm; the angle of the neck in relation to the aneurysm and its implications for fixation of the device; and the length and diameter of the distal landing zone, which, in most cases, is the common iliac arteries.
CTA protocol
There are three goals of CTA of the aorta. First, there should be arterial enhancement of 250 to 300 HU, achieved through rapid injection of contrast material (5 mL/sec). This level of arterial enhancement will enable discrimination of intravascular contrast from aortic calcification and thrombus, while providing adequate contrast for longitudinal resolution of vessels parallel to the slice plane, particularly the renal arteries. The second goal is contrast injection intervals and image acquisition intervals of similar length. The third goal is first-pass cephalocaudad coverage from the supraceliac aorta to the proximal thigh, achieved within a sustainable breath-hold interval.
On a GE 16-channel scanner (GE Healthcare, Waukesha, WI), the imaging protocol would call for 16 detector rows with a collimation of 0.625 mm, resulting in a beam width of 10 mm. Using a pitch of 1.375:1, and a 0.5-second scan rotation, scan speed would be 27.5 mm/sec. Given an average cephalocaudad distance of at least 30 cm from the supraceliac aorta to the proximal thigh, the scan would take approximately 12 seconds. Acquisition interval will vary with required anatomic coverage (depending on patient body habitus) and scan rotation speed.
Once the image acquisition interval has been calculated, the patient's circulation time can be determined through use of either bolus tracking software or by injection of a preliminary mini-bolus and measurement of the time to peak aortic enhancement. This represents the optimal delay between contrast injection and image acquisition. The injection interval can then be made to equal the calculated image acquisition interval (usually approximately 12 seconds).
During image analysis, we use an automated technique to measure diameters and lengths at predefined points along the aorta. This technique uses seed points and automated edge detection to enable centerline tracking of the aorta and the iliac arteries. A slider is moved up and down while the model of the aorta is rotated, selecting specific anatomic points at the level of the renal arteries, the upper and lower margins of the aneurysm, and the iliac bifurcation. The system then calculates diameters (perpendicular to the centerline of the aorta), cross-sectional areas, and the lengths between the selected points.
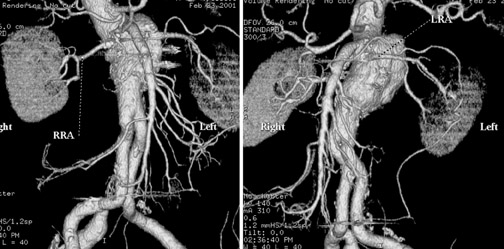
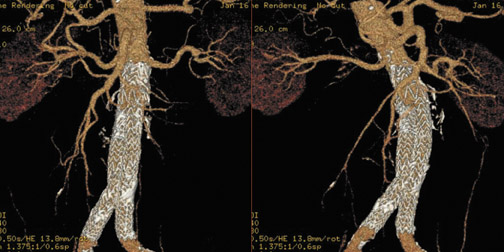
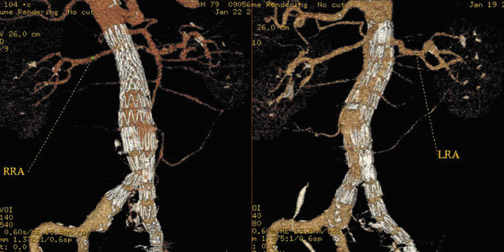
In Figure 1, measurement of the superior neck of the aneurysm, from the renal vascular pedicle to the upper margin of the aneurysm, is defined. The image nicely reveals the aneurysm itself, thrombus and calcification in the wall and, to some extent, atheromatous material. Figure 2 illustrates measurements from the renal vascular pedicle to each iliac bifurcation, enabling an estimate of the length that must be covered by the endovascular stent-graft (not including the superior neck of the aneurysm), and measurement of the distal placement zone.
Contraindications
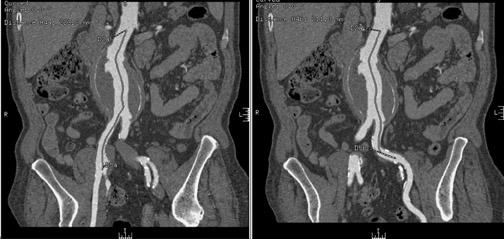
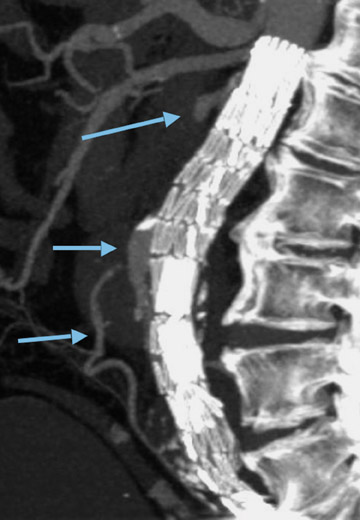
There are several contraindications to aortic stent-graft placement. In the case of juxtarenal aneurysm (Figure 3), the covered portion of the stent-graft would not only exclude the aneurysm but would also overlay and block blood fiow to the renal arteries. Development of fenestrated stent-grafts may overcome this problem in the future.
A short superior aneurysmal neck is a contraindication with some types of stent-grafts, as it does not provide an adequate proximal landing zone. Newer types of stent-grafts that enable transrenal fixation and placement of the upper covered margin immediately underneath the renal vascular pedicle now make it possible to treat such an aneurysm endovascularly, however.
Distal stent-graft placement that would occlude both hypogastric arteries (as would occur if the patient had bilateral common iliac aneurysms extending to the iliac bifurcations requiring distal extension of the stent to the external iliac arteries) represents another contraindication. It would lead to relative ischemia of the pelvis and rectum. This is of particular concern in patients who have stenosis of the superior mesenteric artery.
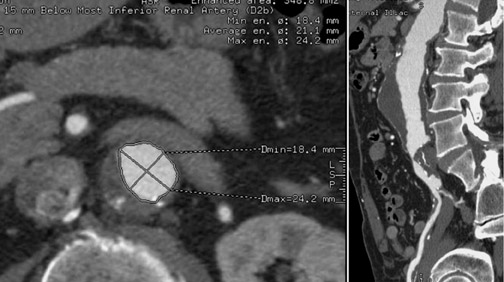
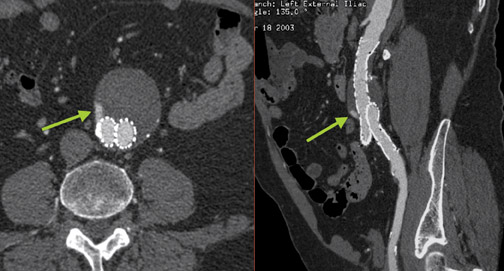
A relative contraindication to stent-graft placement is the presence of large amounts of atheromatous material in the aorta. In Figure 4, atheromatous and atherothrombotic material account for >25% of circumference of the superior neck of the aneurysm. The accompanying orthogonal longitudinal projection reveals a significant amount of atheromatous material in the aneurysm itself.
Concerns also arise in patients who have a history of kidney surgery. For example, in a patient who has undergone nephrec-tomy, it becomes critical that placement of the endovascular graft not traumatize the renal artery supplying the single remaining kidney. Similarly, in a patient with a transplant kidney, passing a large endovascular device through the iliac artery could injure the renal transplant artery.
Devices
The Guidant Ancure system (Guidant Corp., Indianapolis, IN) was one of the earliest stent-graft devices. Although it is no longer available commercially, patients implanted with this device still undergo CT follow-up (Figure 5). It uses barb fixation superiorly and inferiorly and is often supported by internal Wallstents (Boston Scientific, Natick, MA) at the level of the iliac arteries. The aorto-uni-iliac device was used in the early days of stent-grafting in patients with a relatively wide superior aneurysmal neck (these were fabricated locally according to individual patient need). This device had only one iliac limb; therefore, the opposite iliac limb had to be occluded and circulation to the contralateral lower extremity restored with a surgical femorofemoral crossover graft.
The Medtronic AneuRx stent-graft (Medtronic, Inc., Minneapolis, MN) uses friction seal for proximal and distal fixation and accommodates the same aortic dimensions as the Ancure device.
The Gore-Excluder (W.L. Gore & Associates, Inc., Flagstaff, AZ) (Figure 6) has lower-profile introducer (18F) than many other devices and accommodates a 26-mm superior neck. The Zenith stent-graft (Cook, Inc., Bloomington, IN) (Figure 7) uses suprarenal fixation by barb devices, which enables better placement in certain types of patients. The device can be placed in superior necks up to 32 mm in diameter. It also has a bell-bottom configuration that enables it to expand in the iliac arteries for distal placement.
The Medtronic Talent stent-graft, not yet commercially available in the United States, is also able to accommodate a wider superior aneurysmal neck for proximal placement and wider iliac arteries for distal placement.
Complications
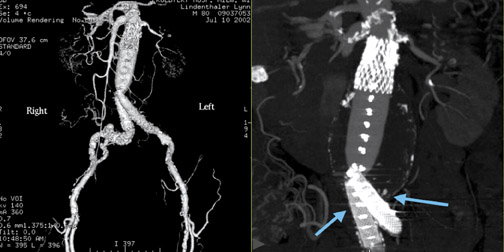
Two major complications of endovascular stent-grafting are atheroembolism during the procedure and Type I endoleak. A Type I endoleak results from inadequate attachment of the stent-graft at either its upper or lower margin (Figure 8). 4 This complication requires early treatment.
A Type II endoleak (Figure 9) or refiow endoleak results from infiow from either the inferior mesenteric or lumbar arteries. The infiow maintains an elevated pressure in the aneurysmal sac, which prevents it from becoming smaller over time, or even causing it to enlarge.
A Type III endoleak is caused by a defect in the graft or to modular disruption. A Type IV endoleak is due to fabric porosity, and is only a potential issue in the initial period after placement.
Generally, patients are imaged within 1 week of stent-graft placement; again at 3, 6, 12, and 18 months; and annually thereafter. During postendograft evaluation, patients have a precontrast imaging study first, so that calcium in the organized wall of an aneurysm sac can later be differentiated from contrast material in the aneurysm sac, which would indicate an endoleak.
Patients then undergo a timed first-circulation study. This yields aortoiliac CTA images and enables measurement of both stent-graft dimensions and the distance between the stent-graft and related anatomy. For example, we may measure clearance between the renal vascular pedicle and the upper margin of the stent-graft, as well as between the lower margin of the stent-graft and the iliac bifurcation to make sure that there has been no interval migration of the stent-graft. The patient also undergoes an immediately delayed study, which, in combination with the initial first circulation study, enables better detection of Type II endoleaks.
The CTA displays we use are familiar ones: stacked axial scans, multiplanar reformations, maximum-intensity projections, volume-rendered displays, curved planar reformations, and quantitative vessel measurements.
Endoleaks can be treated by translumbar needle insertion into the aneurysmal fiow channel. Another option is selective transfemoral catheterization, either using a perigraft approach to catheterization of the lumbar artery, or direct catheterization of the superior mesenteric artery, followed by catheterization of the inferior mesenteric artery. Acrylate injection is used to occlude the perigraft aneurysm sac.
Conclusion
CT angiography is a robust and reproducible technique that provides both three-dimensional imaging and quantitative information critical to the pre- and postintervention evaluation of patients who are candidates for aortic endovascular stent-grafts.
Discussion
LEO P. LAWLER, MD, FRCR: Dennis, thank you; that was very good. There is one area I was wondering about, though. When you are following up, some of you do a noncontrast study, then an arterial-phase study, and then a delayed study. Although I think the multidimensional approach can help in terms of assessment of either stent integrity or migration, certainly in the coronal images, what do you think about the idea that one should really strive to use larger collimation, higher pitch, much lower dose, and limit your z-axis for the noncontrast study and the postarterial phase? In truth, do you really need to have the same kind of coverage? There is at least one paper showing that the dose differences are huge if you can limit yourself that way. But, of course, you are going to be left with 5-mm collimation and so forth.
W. DENNIS FOLEY, MD: Actually, we use thin section only for the angiographic phase and thicker sections for the precontrast and immediate delayed postcontrast acquisition.
LAWLER: Right. But they all have metal components for the sites of purchase. Can you standardize?
BRIAN R. HERTS, MD: Do you have problems trying to distinguish between contrast and calcification in the wall of the aneurysm on the bolus phase if you are using thick sections on the precontrast image?
FOLEY: It is still a pretty good registration. So, yes, we used to look at those images at 5-mm and now at 2.5-mm image thickness for the precontrast and immediate delayed postcontrast studies. We use thin 0.625-mm sections for the first circulation run.
GEOFFREY D. RUBIN, MD: Actually it is for that reason specifically that we use thin sections for the noncontrast phase especially.
At least in our experience, we find that some of this calcification can be pretty tough to separate from small areas of endoleak, and the confident identification of that calcification before the contrast is delivered can sometimes make or break the diagnosis. I think, within the context of radiation dose and exposure, you have to look at your demographics. We are dealing with people who have almost zero probability of developing a radiation-induced neoplasm in their life, especially people with aortic aneurysms. So I really do not see dose as a consideration at all for these people.
HERTS: I find that it is also easier for the technologist to set up the same scan range three times.
LAWLER: I think that is probably true. On the other hand, when one is arguing over "is it calcium or is it contrast," you are generally dealing with something that is very small. Since the majority of endoleaks actually resolve themselves in 6 months or so, I wonder whether we should really be so worried about them in terms of chasing them, especially the small things, to determine whether there is just a little bit of contrast or is it a little bit of calcium. I understand if it is large and the leak is coming back from the lumbar or something. I find they are fairly easy to distinguish.
RUBIN: What is interesting, at least in our experience, is that the actual size of the endoleak, particularly if it is a type II endoleak, does not necessarily associate with the likelihood of a sac shrinking or not. So if the sac does not shrink, even the smallest of endoleaks has to be suspect and of concern, whereas if you have a large endoleak but the sac is shrinking, then the significance of that endoleak is not necessarily so great. I think it is important to keep our eye on the ultimate outcome here, and that is the sac shrinkage and, ultimately, nonrupture.
LAWLER: But do you also find that with sac stability that lack of growth rather than shrinkage is the more common finding? Or do you find most of your sacs shrink?
RUBIN: That is a good point. Actually the likelihood of shrinkage seems to be device-dependent. Ultimately, you like to avoid growth and a lot of them will stay stable.
So what I meant to imply is that with some of these type II endoleaks, even if they continue to grow, that growth is not necessarily associated with the size of the endoleak. This issue of sac shrinkage and device-dependence is something that I think we will need to learn more about. There actually have been some reports of rupturing sacs in the absence of any endoleak. And people are beginning to implicate ePTFE over Dacron as being a semiporous material that allows hygromas to develop in the sac, which results in the so-called endotension phenomenon. In some cases, they have gone on to rupture. Although in that case, the question is what is really rupturing and does it have the same impact.
LAWLER: Dennis, do you believe that endotension exists?
FOLEY: We have had a case similar to what Geoff is describing in which we could not find a cause, yet the aneurysm sac expanded.
U. JOSEPH SCHOEPF, MD: So what is the protocol in your institution? How often do you image those patients and how do you react if you see the sac growing or not?
FOLEY: We image the patients immediately post stent-graft and then at 3, 6, 12, and 18 months and 1 year intervals after that. But if they have an endoleak even in the second year, then I would go with 6-month intervals rather than the 1-year intervals. If a patient really is a critical issue, then I will go with 3 months. That patient I mentioned who had endotension got converted to an open repair.
HERTS: Dennis, I have two questions. You have great information there. First, can you tell us all what images and data you are providing to the surgeon? My second question is one that I hear a lot of radiologists ask at meetings. When you are performing a CTA study, do you look at the other organs? Should we report the full abdominal and pelvic CT if we are only looking at the aorta?
FOLEY: The answer to the second question is yes. We are obligated to look at everything, although we make the disclaimer that it is based on an arterial-phase acquisition. Some things may be missed but, yes, we certainly do look.
In response to your first question, we provide a lot of quantitative information. Although there is a question of how much of that is really utilized.
I try to keep it to what I demonstrated; ie, the superior neck measurements of length, diameter, and angle (ie, angle in relation to aneurysm), and the length and diameter of the common iliac arteries. That is rather critical information. Otherwise, you could build up a large table of information that just sits there not being used.
ELLIOT K. FISHMAN, MD: Let me ask one last question about selection of contrast agents. Many of your images showed patients with aortic aneurysms, or in the age group in which they have atherosclerotic disease throughout many vessels, and many of them had decreased renal size. In choosing agents, is the se-lection of an isosmoloar agent in those patients something to consider?
FOLEY: One of the issues is that we do get patients with marginal renal function. On the other hand, the amount of contrast we give is relatively small.
So you can do a study with 60 to 70 mL of contrast plus a preliminary mini-bolus. We do use the isosmolar contrast agent when their creatinine level is >2, which I think other people would suggest. But, otherwise, <2 we are not really concerned about the type of contrast agent because we are using a relatively small amount.
FISHMAN: Right. I guess the question to follow it up might be: Is there any concern with the smaller volumes of contrast? I know in terms of doing measurements, it is not an issue. But following a patient with suspected endoleak, is there some volume of contrast that makes you feel comfortable that you are picking up those leaks?
FOLEY: You may have hit me on a weak point there because, yes, we do use the same technique. It is possible, and I suspect you may be right, that particularly with that second-pass immediate delayed imaging sequence, you probably are more sensitive if you inject more contrast to begin with.