The opacified paranasal sinus: Approach and differential
Images

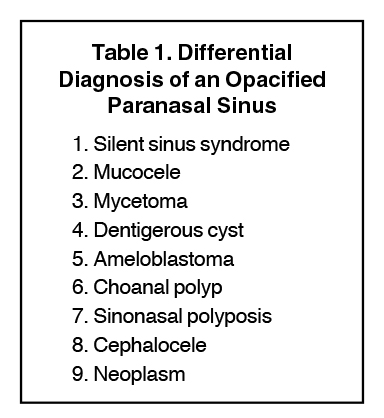
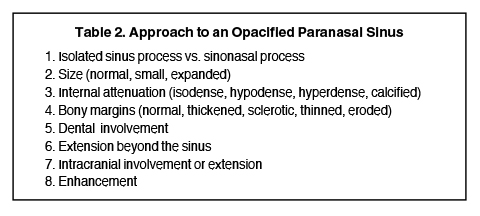
Sinonasal inflammatory disease with sinus ostial obstruction is a very common cause of an opacified paranasal sinus. However, there is a differential for an opacified paranasal sinus (Table 1). This article will present the typical appearances of these entities and demonstrate the benefit of a systematic approach in the evaluation of an opacified sinus (Table 2).
Inflammation
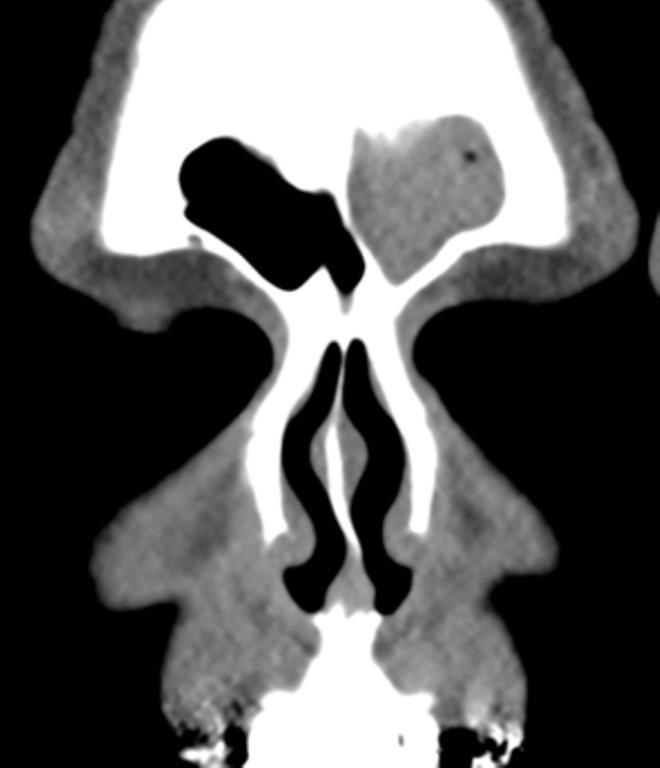
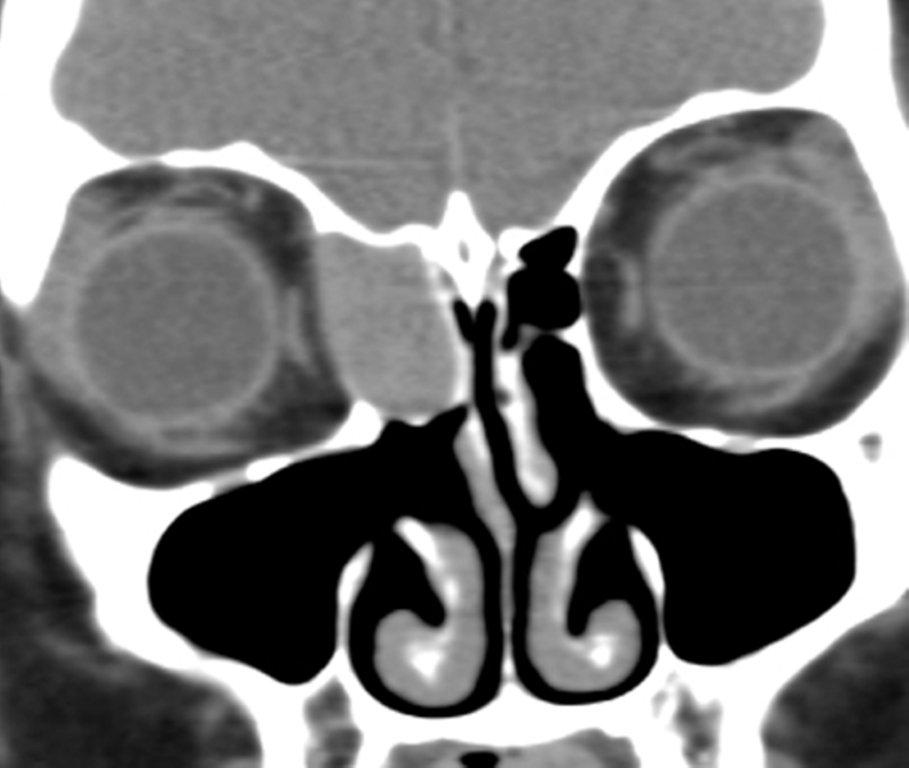
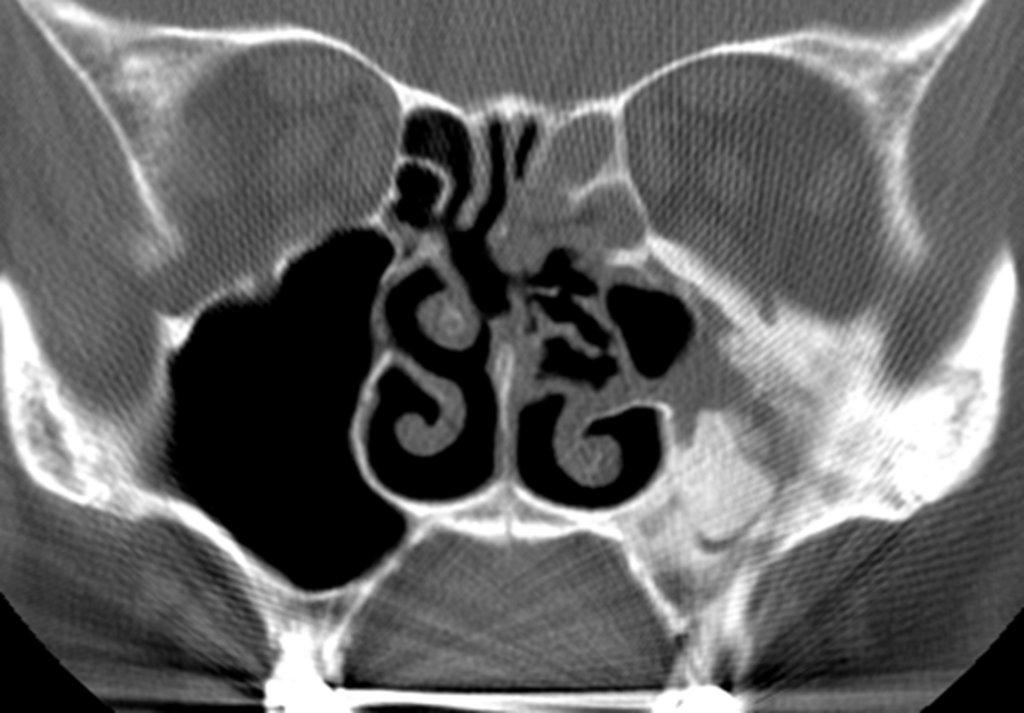
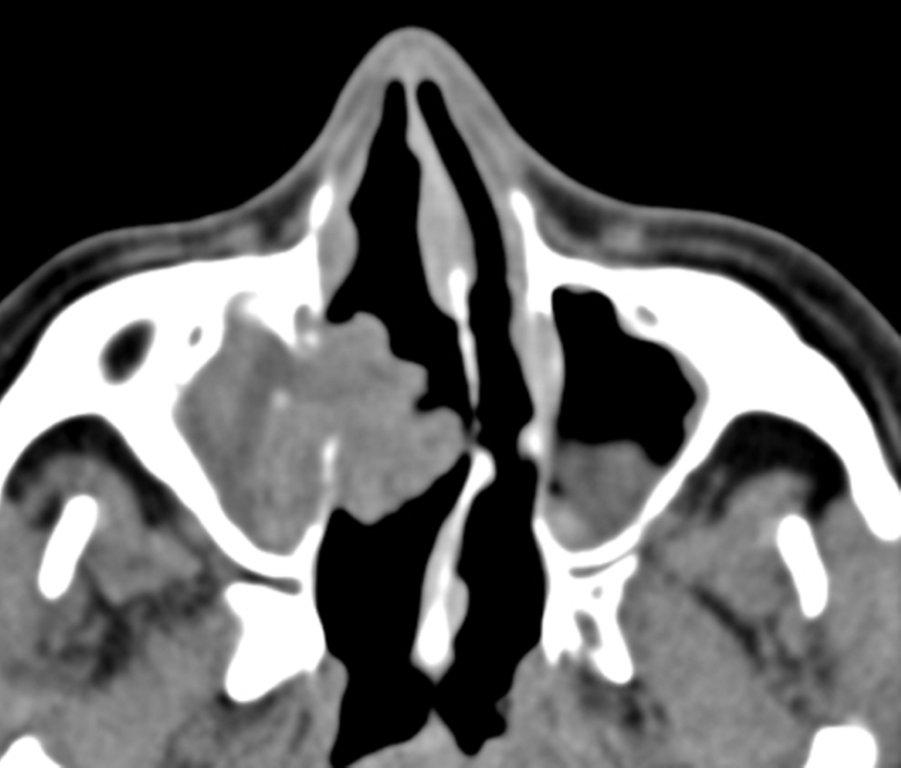
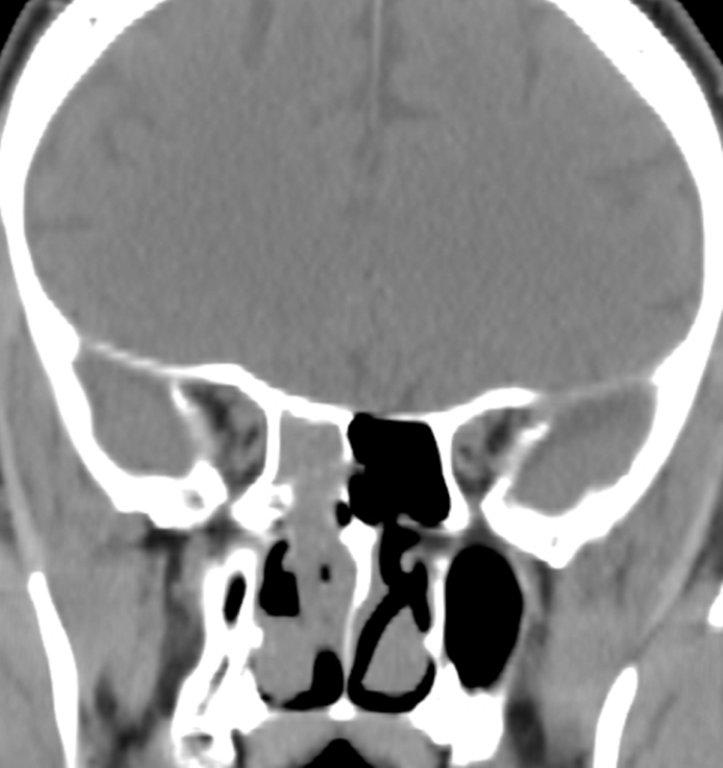
Sinonasal inflammatory disease with sinus ostial obstruction is a very common cause of an opacified paranasal sinus. An air-fluid level suggests acute sinusitis; in chronic sinus disease, one may see mucosal thickening and sclerosis of the bony sinus walls.1 The sinus is normal in size. There are certain recurring patterns of inflammatory sinus disease that may be seen on sinus computed tomography (CT).2 These include: the infundibular pattern, with inflammation of the maxillary sinus and opacification of the ipsilateral ostium and infundibulum; the ostiomeatal unit pattern, with inflammation of the ipsilateral maxillary, frontal and ethmoid sinuses and occlusion of the middle meatus (Figure 1); the sphenoethmoidal recess pattern, with obstruction of the sphenoethmoidal recess and inflammation of the ipsilateral posterior ethmoid and sphenoid sinuses; the sinonasal polyposis pattern, which is characterized by the diffuse presence of polyps in the paranasal sinuses and nasal cavity; and the sporadic pattern, also termed unclassifiable, which is diagnosed when there is random sinus disease not related to ostial obstruction or polyposis. The most commonly occurring patterns are the infundibular, ostiomeatal and sporadic. Knowledge and identification of these patterns may be helpful in surgical planning.
Silent sinus syndrome (SSS)
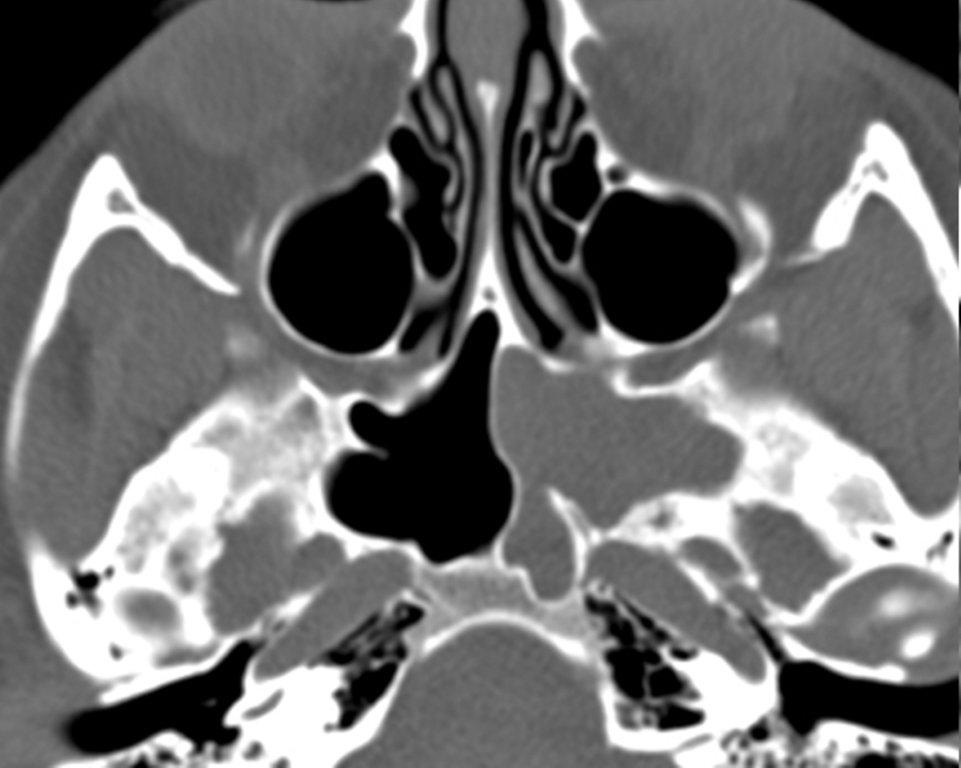
Both silent sinus syndrome (SSS) and the mucocele, which is discussed in the next section, are characterized by abnormal sinus size, with reduced sinus volume in SSS and sinus expansion in mucocele.
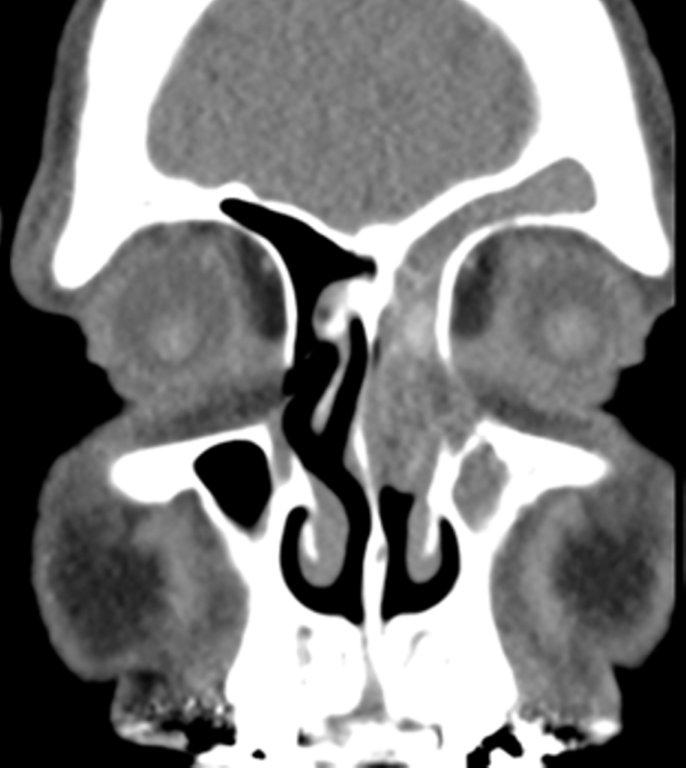
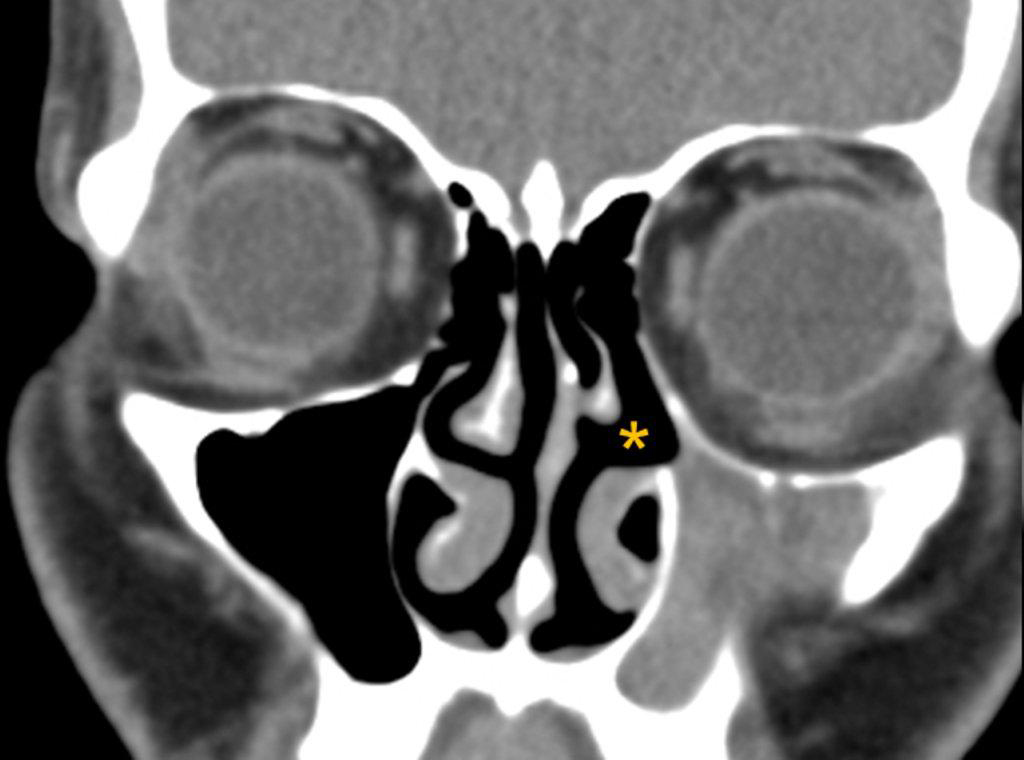
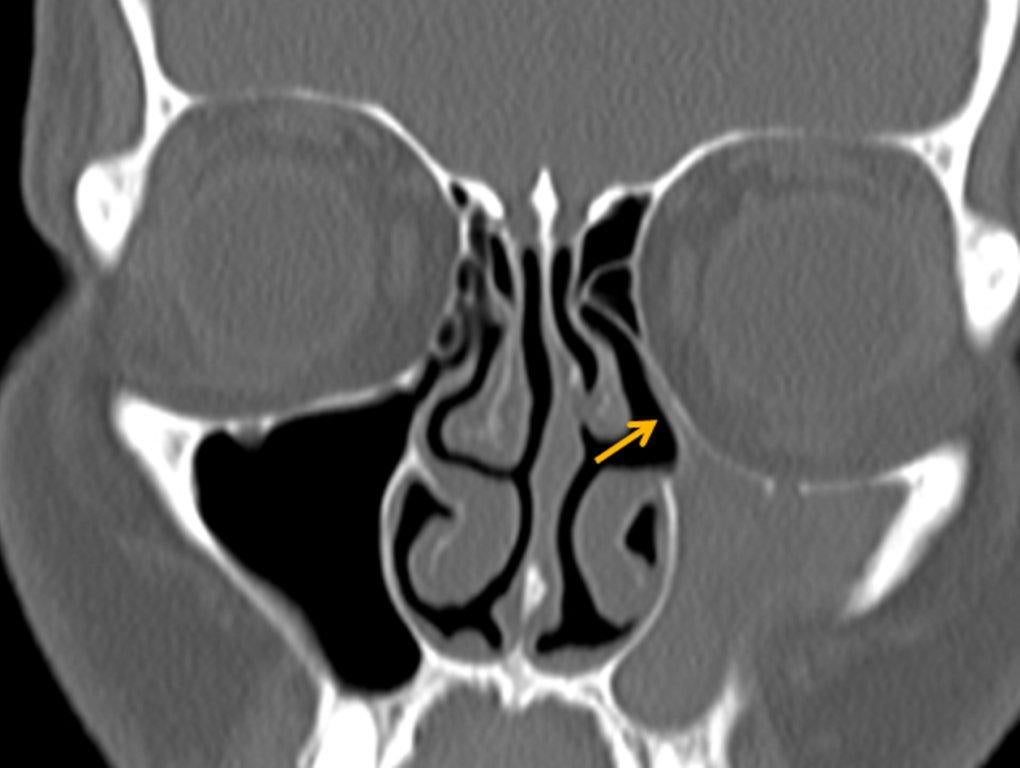
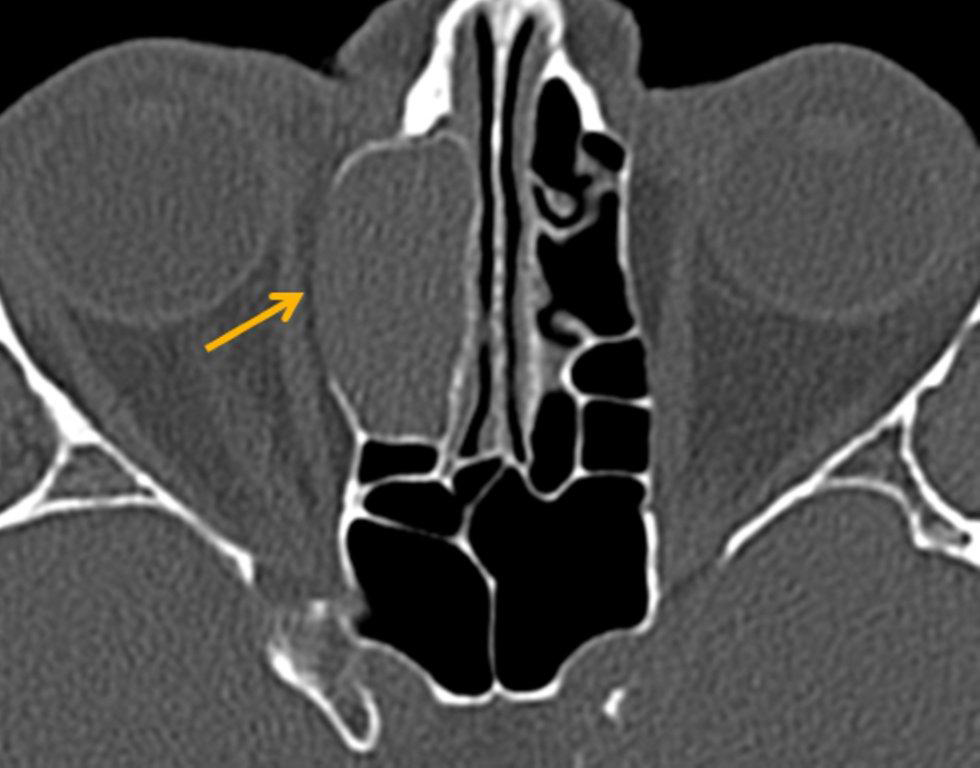
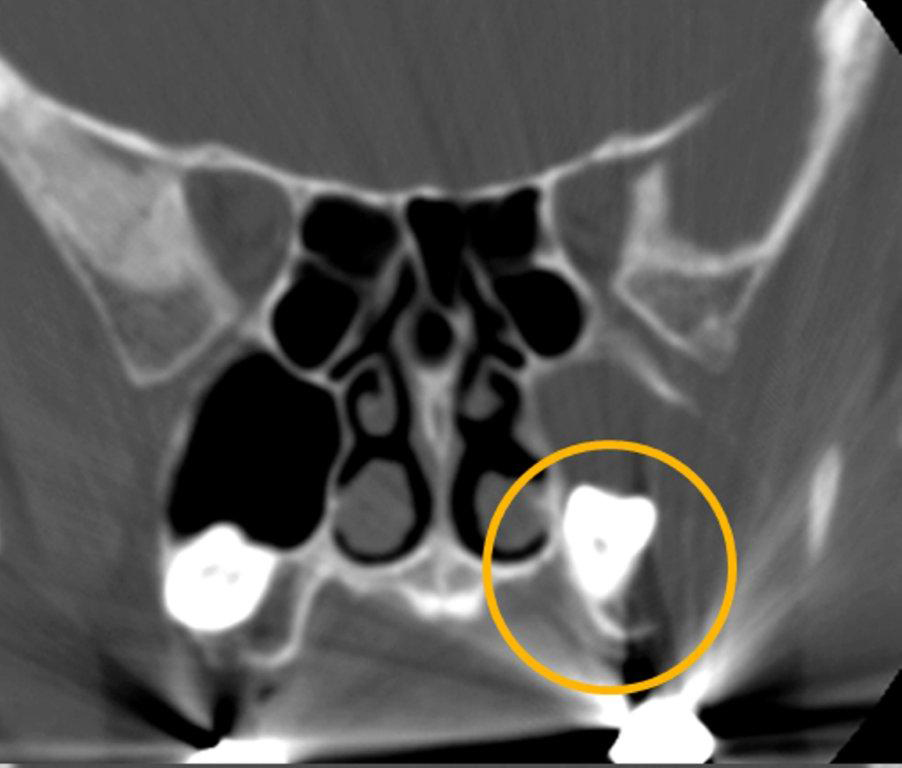
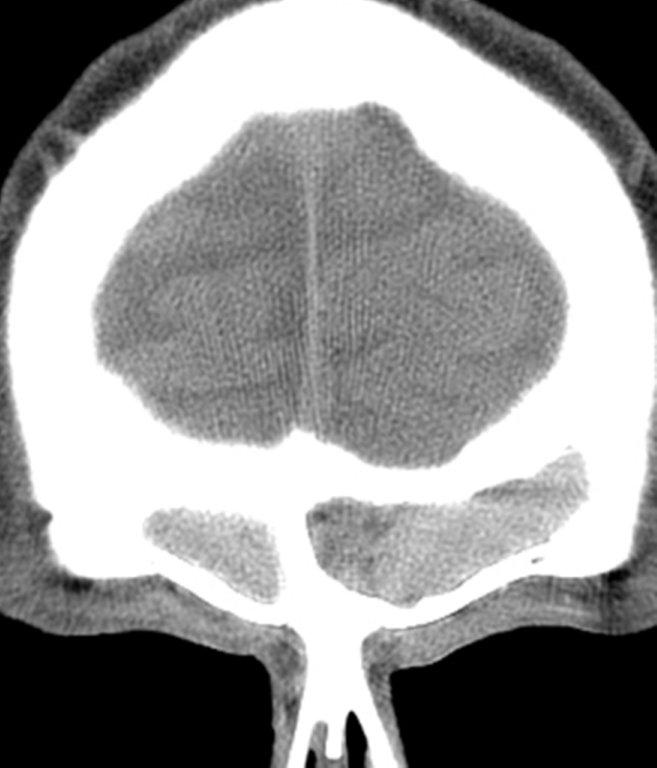
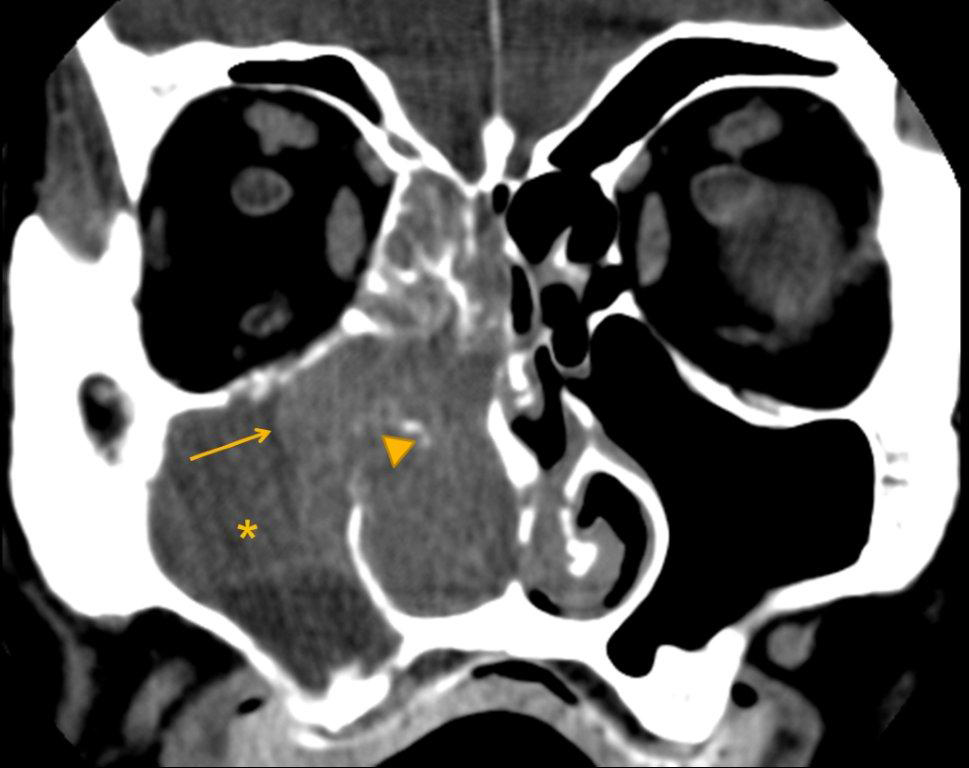
The term “silent sinus syndrome” is characterized by unilateral progressive painless enophthalmos, hypoglobus and facial asymmetry due to chronic maxillary sinus atelectasis.3 The etiology is most likely obstruction of the ipsilateral maxillary ostium, whether it is idiopathic or (less likely) postoperative in nature;4,5 there is subsequent hypoventilation and negative pressure of the sinus and resultant remodeling. There are no significant sinus symptoms—hence the “silent” nature of the syndrome. Alternative terms for the condition include the “imploding antrum syndrome”6 and chronic maxillary sinus atelectasis.7
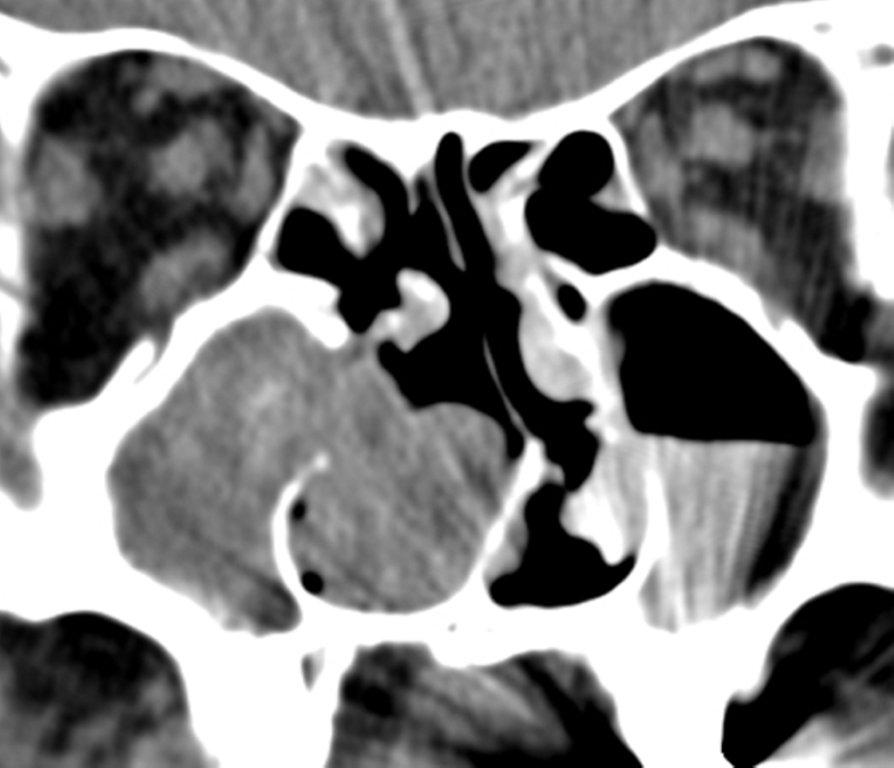
Although diagnosed clinically most often, the imaging features are characteristic. These include diminished sinus volume with retraction and inward bowing of the maxillary sinus walls, downward bowing and thinning of the orbital floor and increased orbital volume, near-to-complete sinus opacification, lateral retraction of the uncinate process with apposition of the uncinate against the inferomedial orbital wall, enlargement of the middle meatus and an increased retroantral fat pad (Figure 2).
Although the classic description of SSS includes enophthalmos, cases with lateralized uncinate processes and increased orbital volumes on CT scans, but without clinical enophthalmos, have been reported.8 These may represent early cases of SSS prior to development of clinical orbital findings.
The definitive treatment is surgical with endoscopic uncinectomy and opening of the maxillary ostium.9 This will arrest disease progression; sinus volume typically stabilizes, although it may improve slightly or regain near normal configuration. Orbital floor repair may be helpful in cases with diplopia, severe cosmetic deformity or those with little improvement following endoscopic surgery.3
Most certainly an acquired condition, SSS should be distinguished from maxillary sinus hypoplasia, which is characterized by lack of pneumatization laterally into the malar eminence and inferiorly into the maxillary alveolar ridge.3
Mucocele
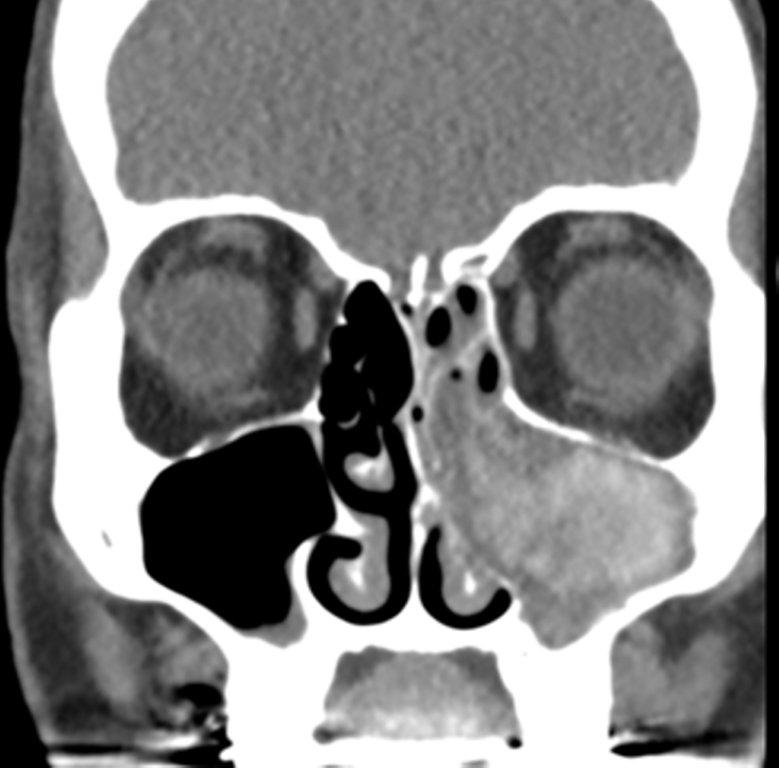
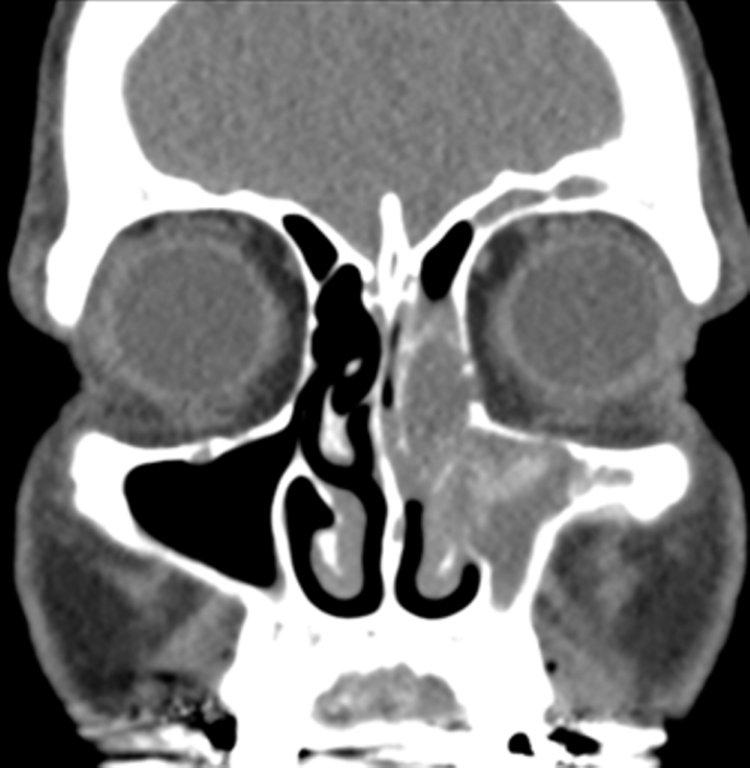
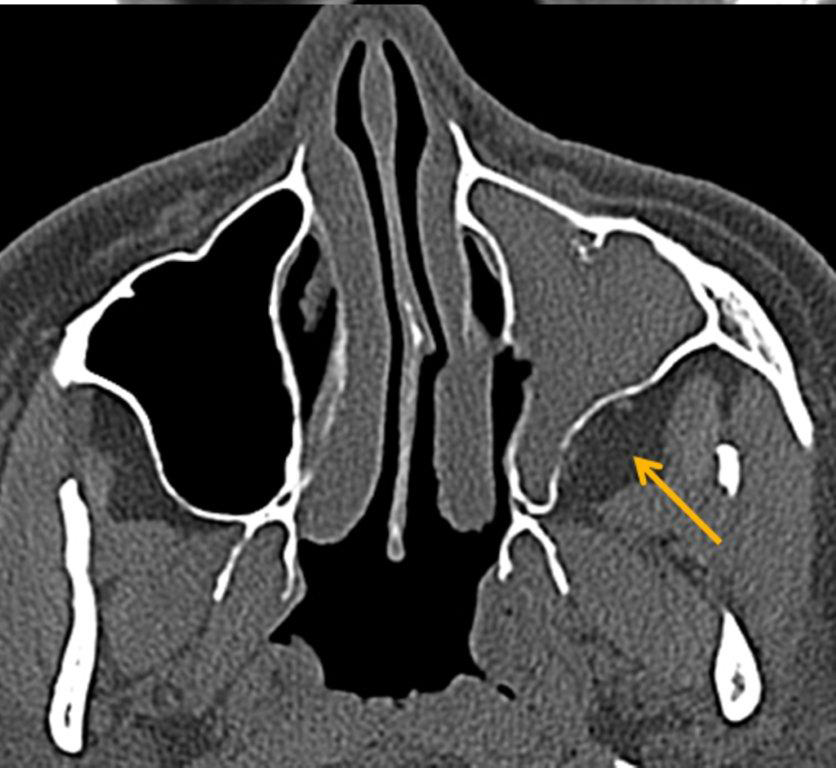
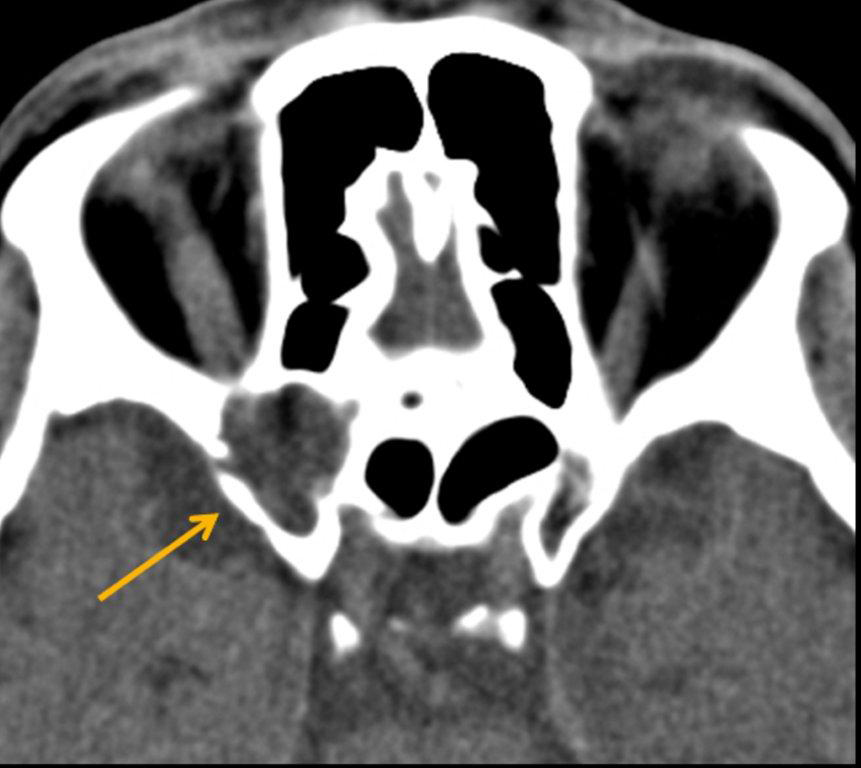
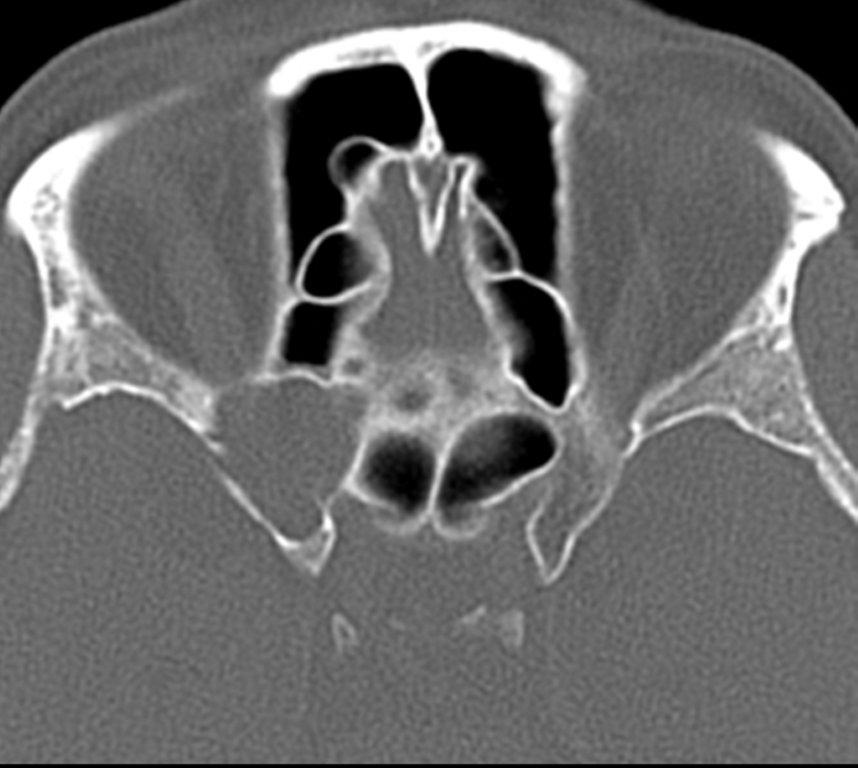
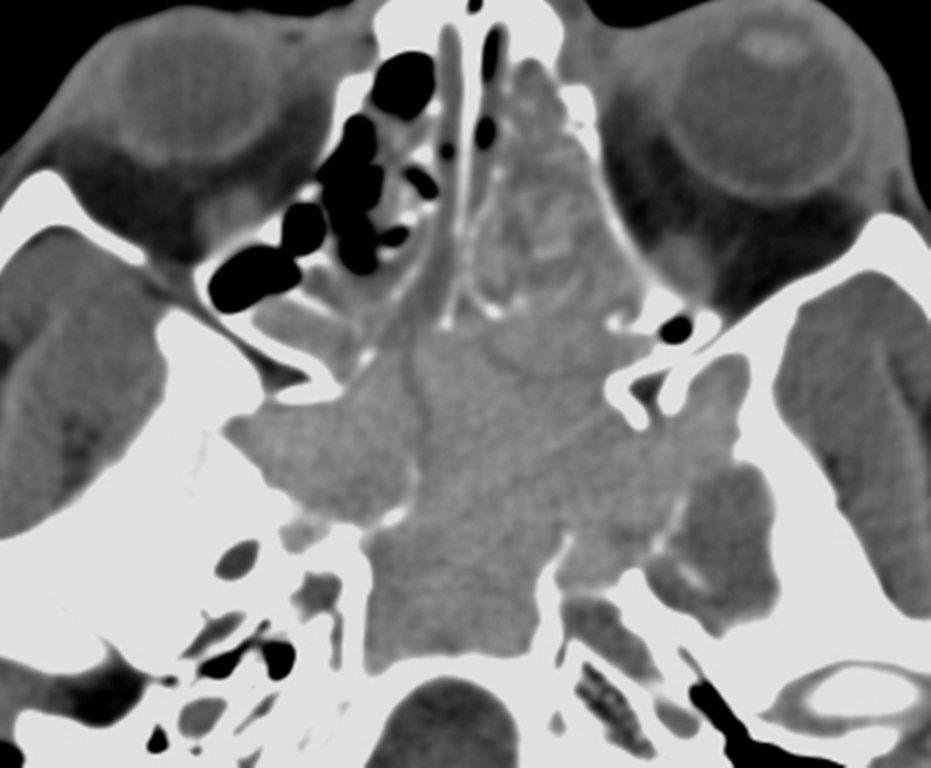
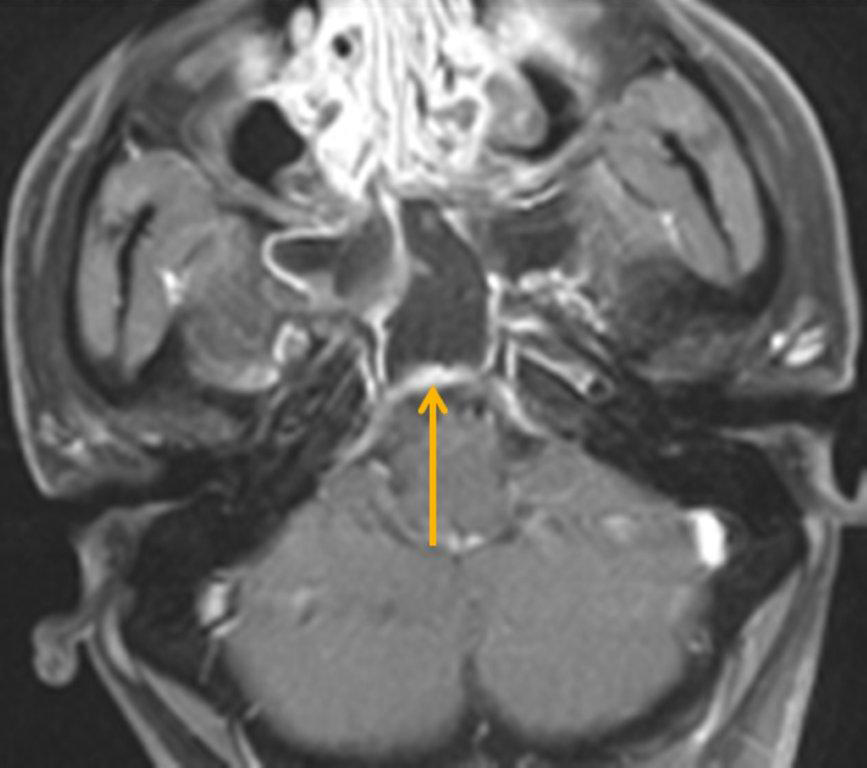
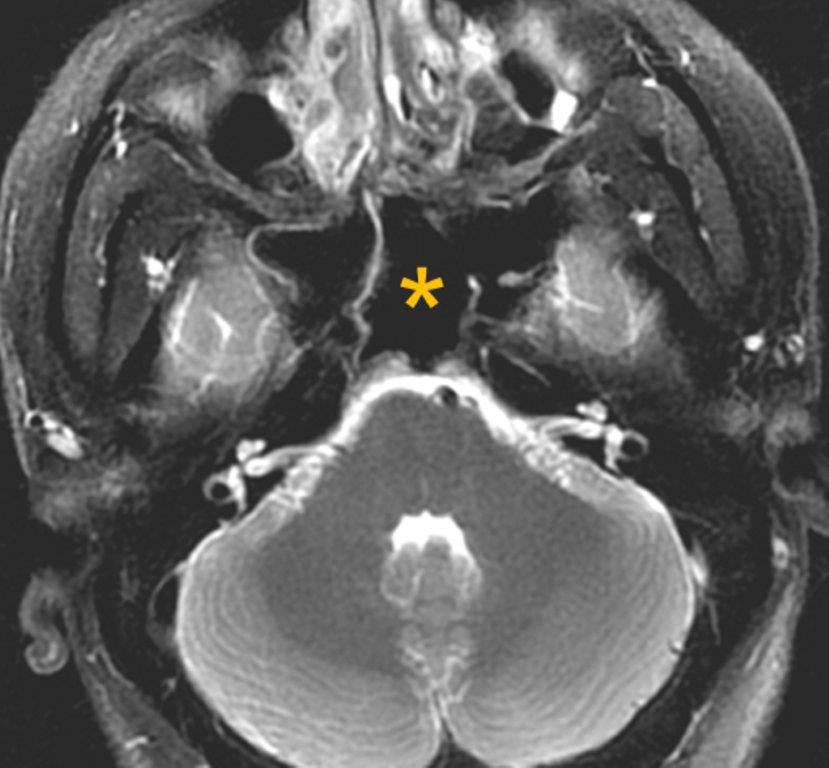
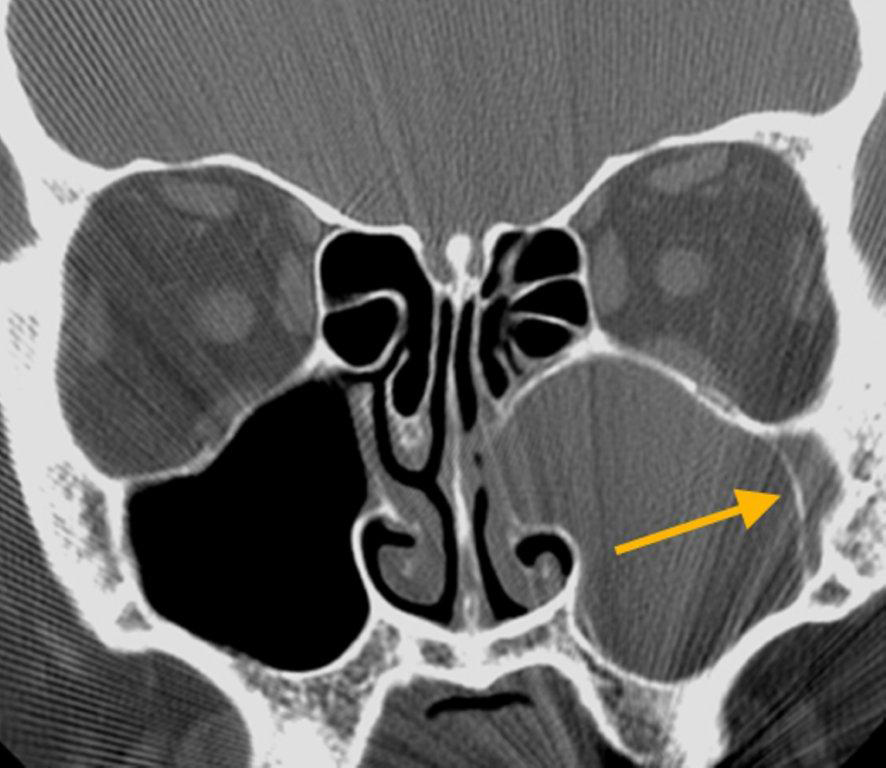
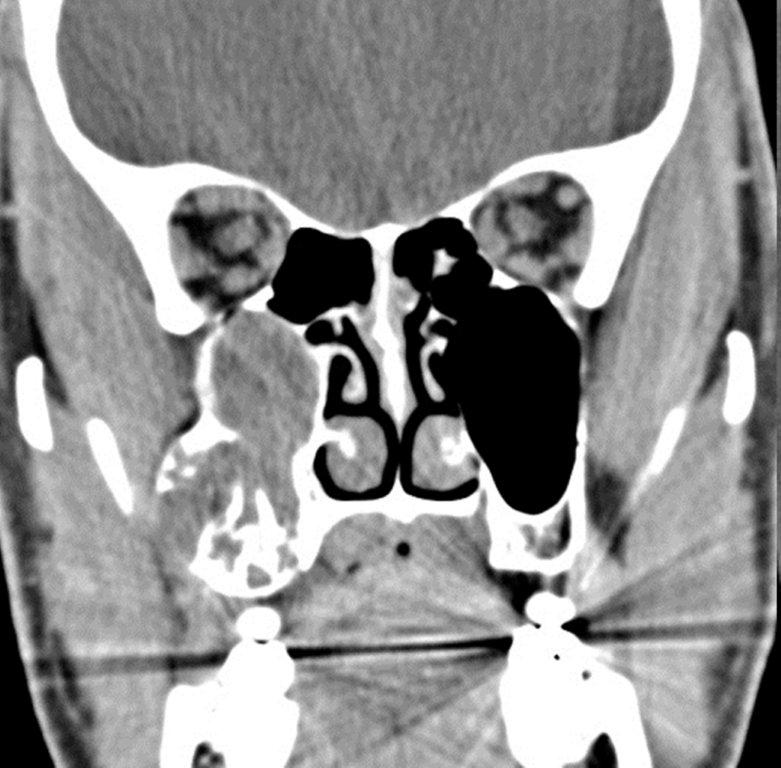
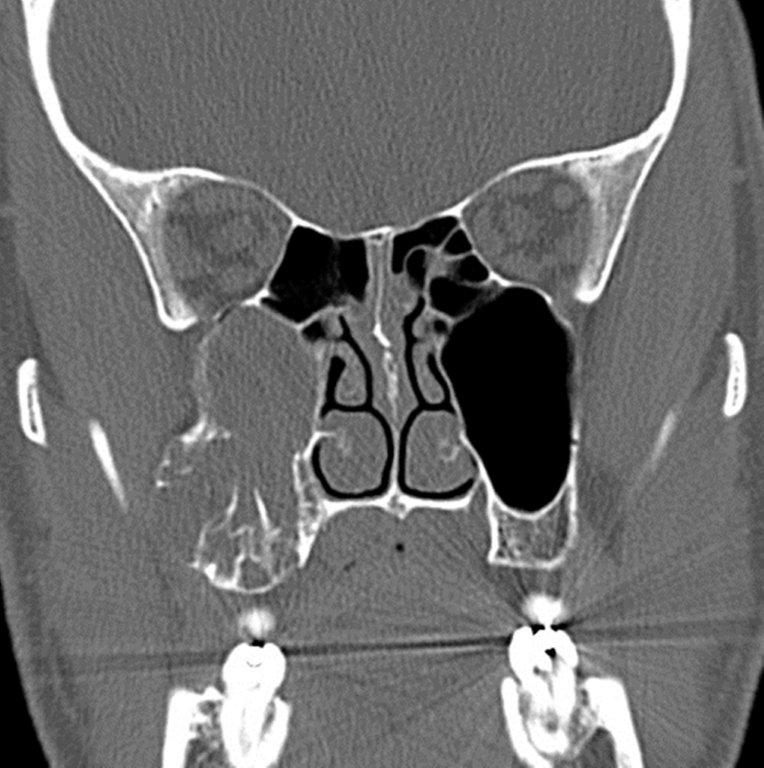
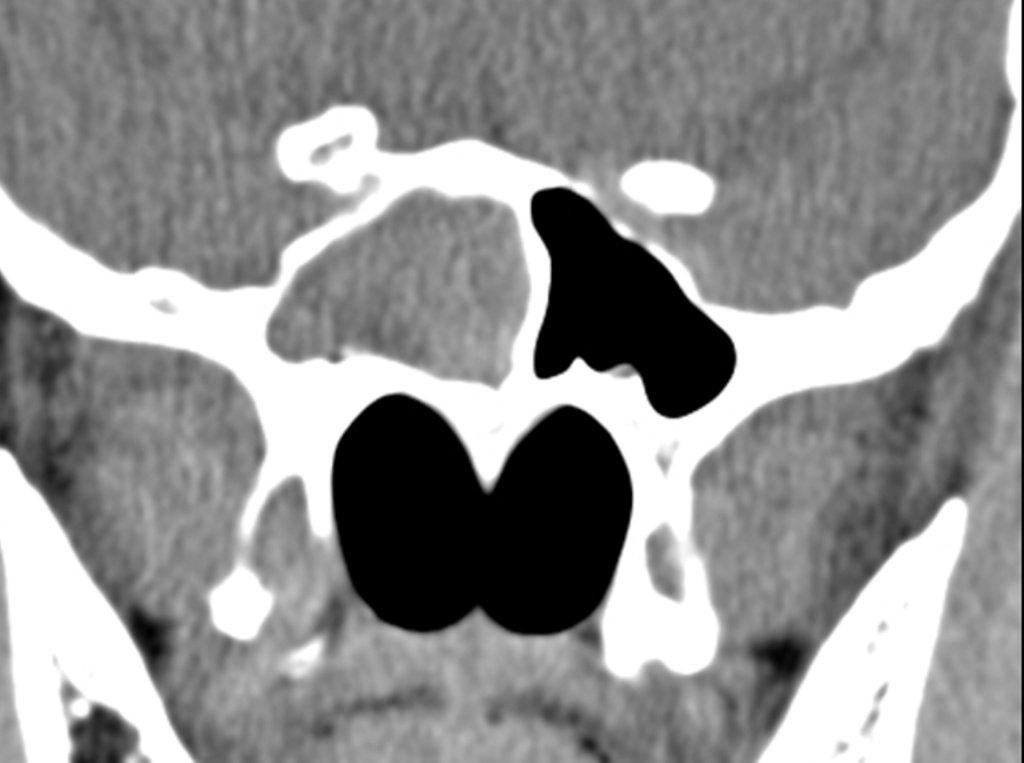
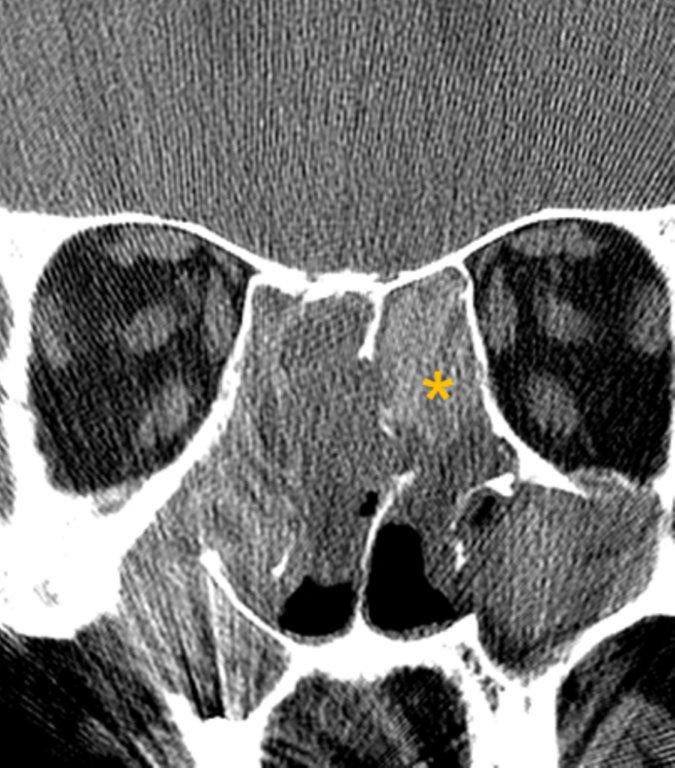
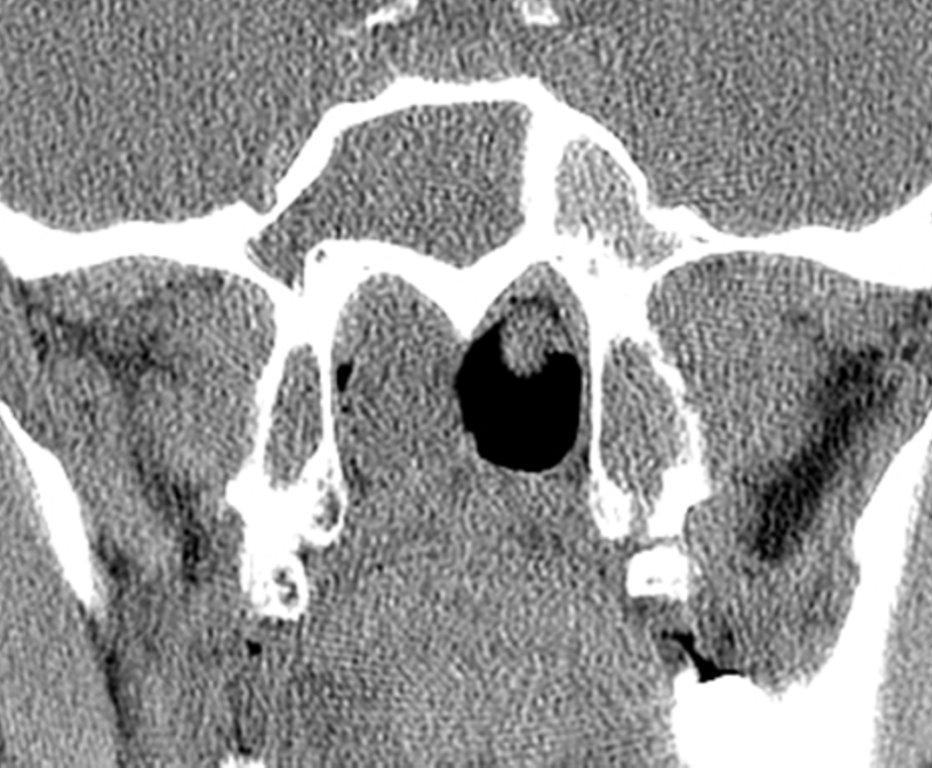
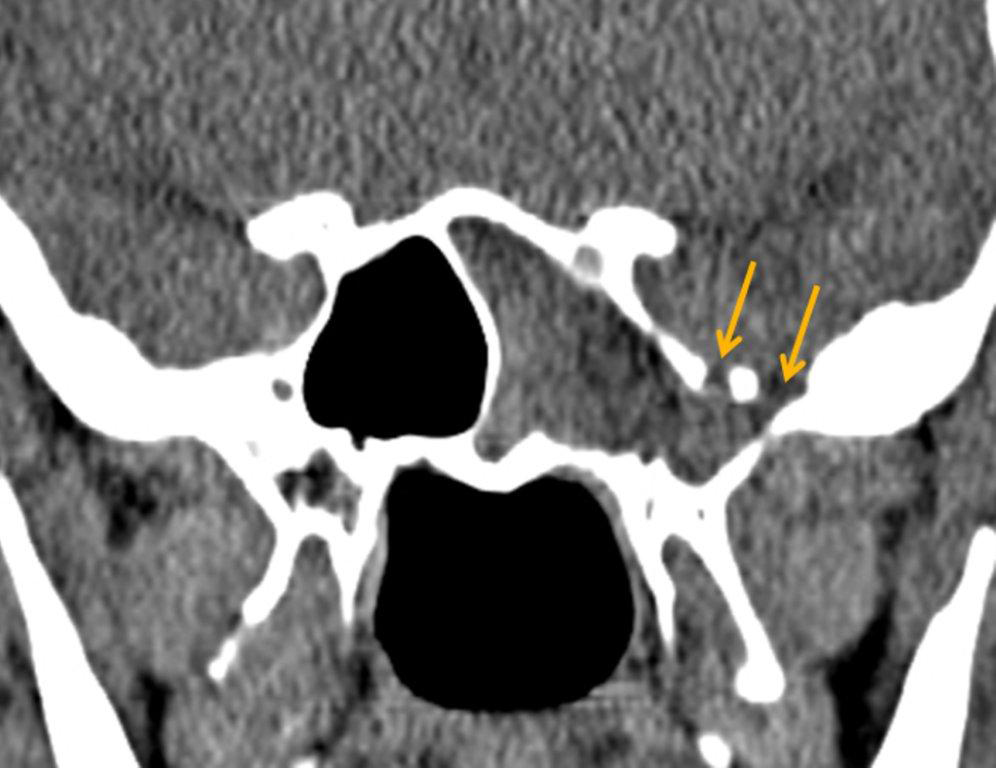
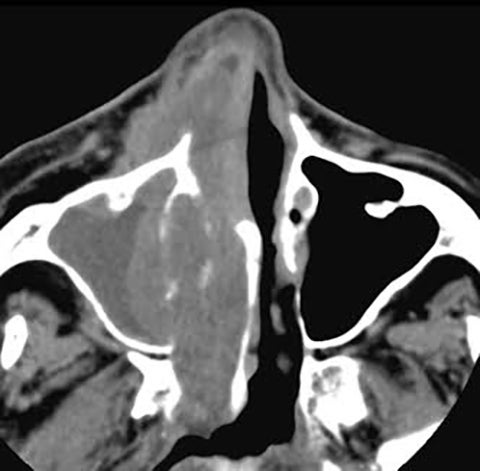
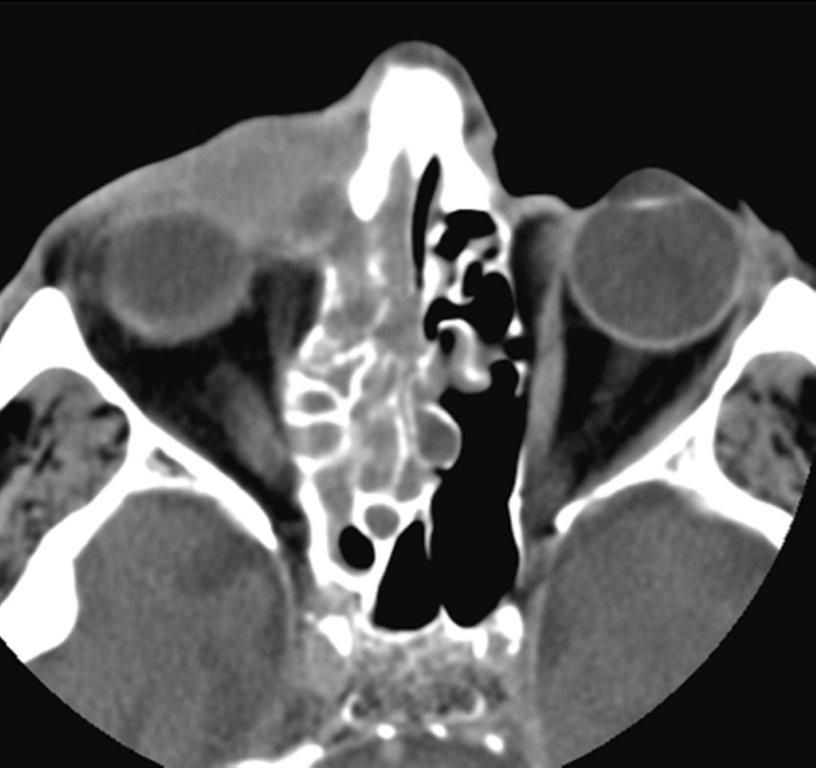
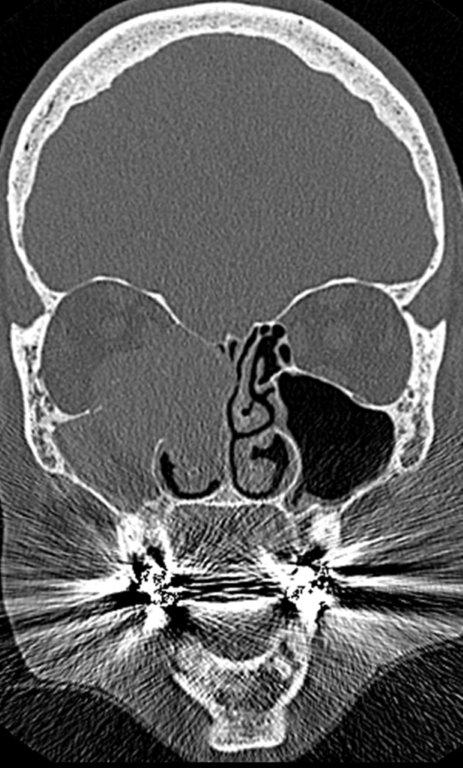
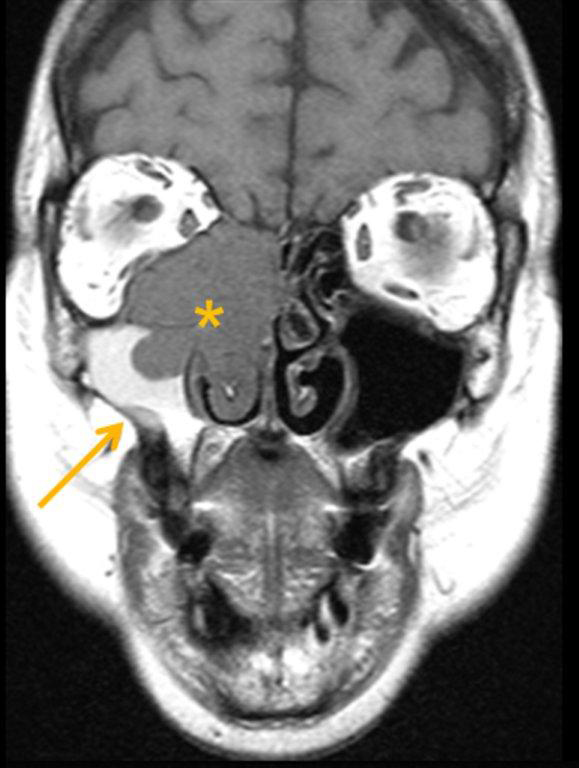
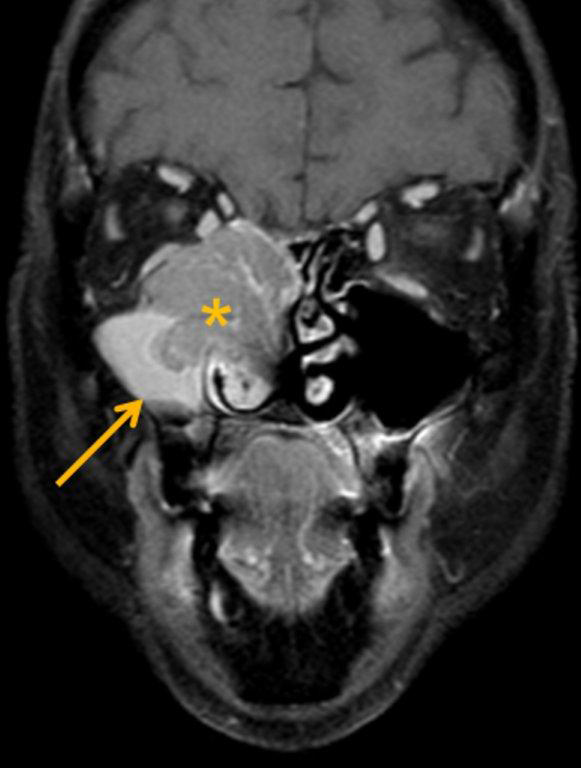
By definition a completely opacified, nonenhancing and mucus-filled expanded sinus, a mucocele is the most common expansile mass of a paranasal sinus (Figure 3). Most often secondary to an obstruction of the sinus ostium, mucoceles may also result from surgery, osteoma or prior trauma; this is especially true of frontal sinus mucoceles.10 The frontal sinuses are most frequently affected (60-65%), followed by the ethmoid (20-30%), maxillary (10%) and sphenoid (2-3%).11 Sinus expansion may result in intracranial and intraorbital extension, with mass effect on adjacent structures. Mucoceles can also occur in isolated sinus cells. Optic neuropathy and acute visual loss caused by an isolated mucocele of an Onodi cell have been reported.12 An Onodi (sphenoethmoid) cell is the most posterior ethmoid cell that pneumatizes laterally and superiorly to the sphenoid sinus and is intimately associated with the optic nerve (Figure 4).13
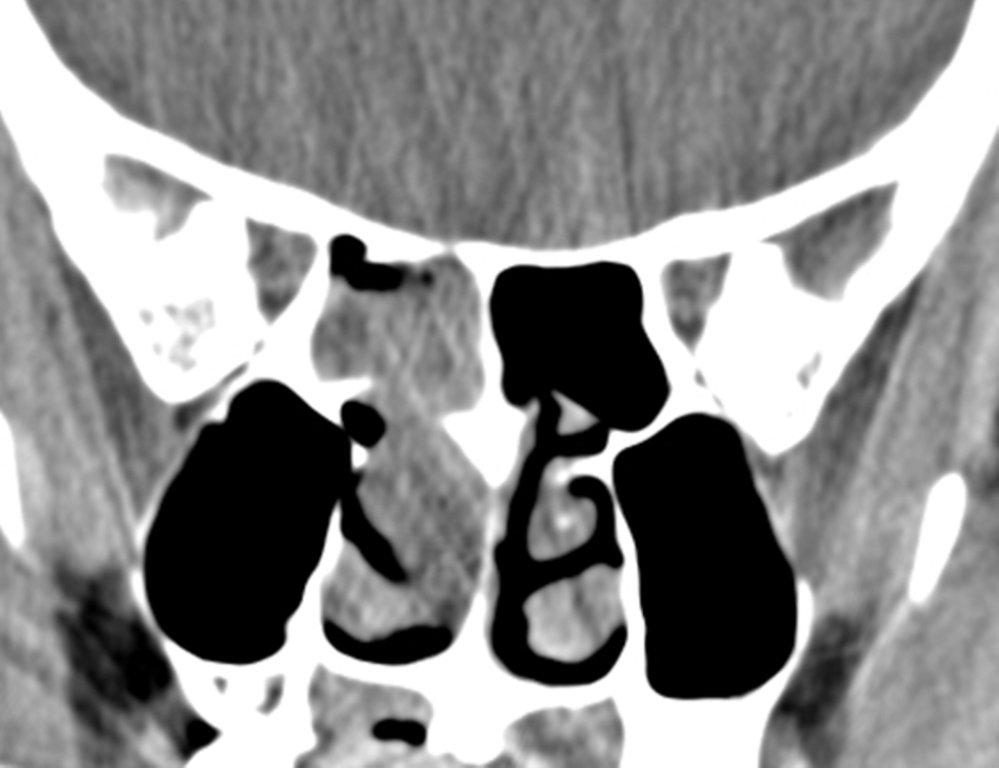
On CT, mucoceles typically appear homogenous and isodense relative to brain tissue. The remodeled sinus walls may be of normal thickness, although thinning, erosion and dehiscence can also be seen. Thin rim enhancement is seen in infected mucopyoceles.14
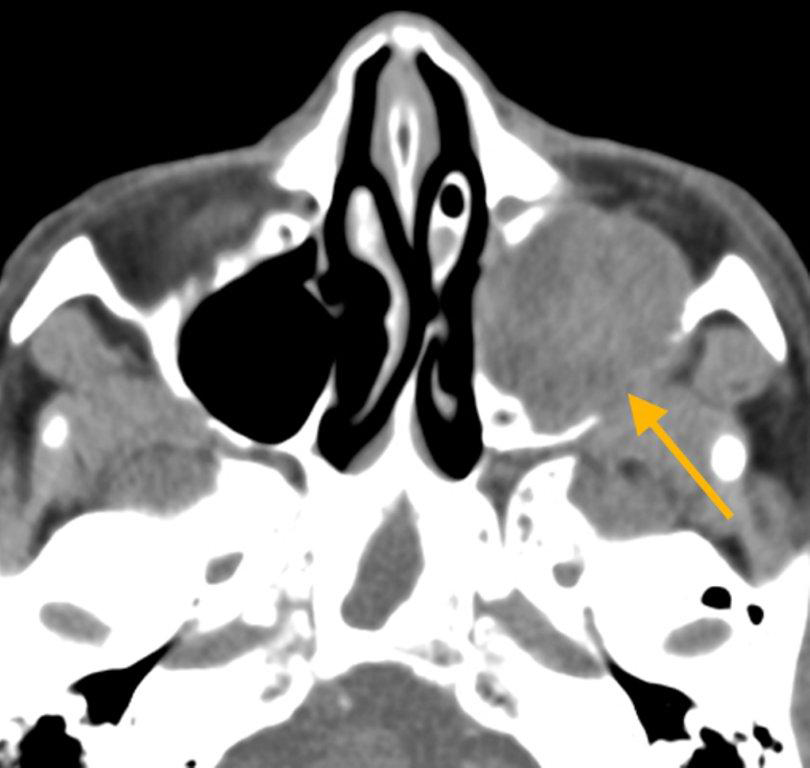
The appearance on MRI is variable depending on the composition of the mucocele. Decreased T1 and T2 signal intensity is most consistent with inspissated secretions and there is often increased attenuation on CT. More hydrated and proteinaceous secretions are associated with T1 and T2 hyperintensity, with isodense or hypodense appearance on CT.15 Enhanced MR examination may help distinguish mucocele and neoplasm, with thin regular peripheral enhancement suggestive of mucocele, and solid or homogenous enhancement typically seen in neoplasm (Figure 5).16
Mycetoma
Mycetoma, also known as a “fungus ball,” is a manifestation of fungal sinus disease. Fungal sinusitis is broadly categorized as invasive or noninvasive, with five major subtypes. The noninvasive subtypes typically occur in immunocompetent individuals and include mycetoma and allergic fungal sinusitis. Acute invasive fungal sinusitis, chronic allergic fungal sinusitis and chronic granulomatous fungal sinusitis constitute the invasive subtypes. The absence of hyphae with the mucosa, bone and blood vessels characterizes noninvasive fungal sinusitis, while invasive fungal sinusitis is defined by the presence of hyphae within the mucosal and submucosal tissues, bone and blood vessels of the paranasal sinuses. Clinical symptoms of mycetoma are either absent or minimal.17
The mycetoma represents a densely packed collection of fungal hyphae with no allergic mucin. It appears as a hyperdense mass with internal calcifications within the sinus on unenhanced CT (Figure 6); the inflamed mucosa peripherally may be hypodense. The mycetoma tends to involve a single sinus, most commonly the maxillary, with the sphenoid sinus affected less frequently. The bony walls may be thickened and sclerotic or expanded and thin.
On MRI, a mycetoma most often appears dark or hypointense on T2-weighted images, likely reflecting the combination of lack of water and the presence of calcification and paramagnetic materials.17,18 Inspissated, proteinaceous secretions associated with chronic obstructive non-fungal sinus disease may also demonstrate increased attenuation on unenhanced CT and very low signal intensity on both T1- and T2-weighted MR images.19
Dentigerous cyst
Both dentigerous cysts and ameloblastomas, which are discussed in greater detail in the next section, originate in the bony maxilla and may then secondarily involve the adjacent maxillary sinus.
The dentigerous cyst is the most common type of a developmental odontogenic cyst. They are typically solitary, and 75% of lesions are located in the mandible. They may be associated with any unerupted tooth, but most commonly with the mandibular third molars. The maxillary canines and maxillary third molars are involved less frequently. A dentigerous cyst that involves a paranasal sinus usually is related to a maxillary canine, with secondary extension into the antrum.20
A dentigerous cyst arising in the bony maxilla outside of the antrum is, by definition, an extra-antral lesion. The demonstration of a thin bony plate representing the floor of the maxillary sinus between the cystic expansile mass and the adjacent maxillary sinus is critical in the identification of the extraantral nature of the lesion.21
On CT, a dentigerous cyst demonstrates an expanded unilocular radiolucent area that is associated with the crown of an unerupted tooth (Figure 7). The border is typically sclerotic and well-defined, although an ill-defined border may be seen with infection.22
Ameloblastoma
Ameloblastoma is the most common odontogenic tumor. Defined as a benign epithelial neoplasm, the tumor is locally aggressive and invasive. Incomplete resection may result in local persistence or recurrence or, rarely, distant typically pulmonary metastases. Interestingly, approximately 50% of ameloblastomas arise from the lining of a dentigerous cyst.23 A unilocular ameloblastoma may be difficult to distinguish from a dentigerous cyst; the presence of a small intracystic mural nodule, seen in ameloblastoma, is helpful in differentiation.21
Like dentigerous cysts, ameloblastomas occur much more commonly in the mandible than the maxilla; only 20% of ameloblastomas occur in the maxilla.22 The premolar-first molar location is common for a maxillary ameloblastoma. These maxillary tumors then easily expand into the ipsilateral antrum or the adjacent nasal cavity.
On CT, an ameloblastoma appears as an expansile cystic, solid or mixed attenuation lesion with scalloped margins. Thinning of the maxillary cortex is often extensive. The lesion may be uni- or multilocular and contain internal septations; the resultant “honeycomb” or ‘bubbly” appearance is typical but not pathognomonic. The solid regions may show contrast enhancement. (Figure 8). Isointense T1 and hyperintense T2 signal on MR imaging with intense inhomogeneous enhancement and nonenhancing low-signal foci internally has been reported with maxillary ameloblastoma.24
Choanal polyp
A choanal polyp is a benign solitary sinonasal mass that originates in a paranasal sinus and secondarily extends into the nasal cavity. The most common type is the antrochoanal polyp, which originates in the mucosa of the maxillary sinus or antrum. The polyp opacifies and slightly enlarges the sinus cavity with no bone destruction. The enlarged antral polyp protrudes through the maxillary infundibulum or accessory ostium into the middle meatus and then the posterior choana, with possible extension into the posterior nasopharynx. Antrochoanal polyps (Figure 9) are rarely bilateral.25
Sphenochoanal and ethmoidochonal polyps are less common than antrochoanal polyps. A sphenochoanal polyp begins within the sphenoid sinus and extends through an enlarged sphenoid ostium into the sphenoethmoidal recess and from there into the choana. The sphenochoanal polyp typically lies between the nasal septum and the middle turbinate (Figure 10); this space is clear with antrochoanal polyps.26
A choanal polyp is hypodense on CT, hypointense on T1-weighted and hyperintense on T2-weighted MR images, with peripheral contrast enhancement. The differential diagnosis of a choanal polyp includes inverted papilloma; these may look identical on unenhanced sinus CT, with characteristic calcification reported in 10% of inverted papillomas (Figure 10). Central or curvilinear enhancement may also be seen.27 The surgical management involves complete removal of the polyp (both antral and nasal parts) and wide middle meatal antrostomy, along with correction of any predisposing anatomic variants.28 It is critical to correctly document the sinus of origin by demonstrating continuity between the choanal polyp and the opacified sinus.
Sinonasal polyposis
Sinonasal polyposis is a typically extensive process with involvement of both the nasal cavity and the paranasal sinuses. By contrast, mucous retention cysts are typically limited to the sinus cavity in location. CT findings of sinonasal polyposis include polypoid masses in the nasal cavity, polypoid soft tissue masses in the sinuses, partial or complete pansinus opacification, and enlargement of the infundibula. Other findings include attenuation of the nasal septal and sinus trabeculae, and opacified ethmoid sinuses with bulging lateral walls. Chronic bony changes with thickening and sclerosis can be seen.29,30 Truncation of the middle turbinate, with absence of the bulbous part of the bony middle turbinate, has been reported as a characteristic ancillary imaging finding in sinonasal polyposis, not seen in other patterns of sinusitis.31 On CT, the polyps are usually hypodense or isodense in appearance; foci of increased attenuation may be seen with inspissation or superimposed fungal sinusitis (Figure 11).The initial treatment of sinonasal polyps is medical management, including intranasal or systemic steroids, and antibiotics. Surgery is reserved for those who do not respond to medical therapy. Medical treatment following surgery is essential in preventing recurrence.32
In patients with known or suspected sinonasal polyposis, unenhanced sinus CT is the examination of choice. Enhanced CT or MRI may be helpful in select cases, such as distinguishing polypoid mucosal hypertrophy from sinus fluid, excluding an obstructing soft tissue neoplasm, and in cases with atypical CT findings and aggressive-looking bone destruction.27,33 Most sinonasal polyps enhance peripherally but may occasionally show solid enhancement.34
Cephalocele
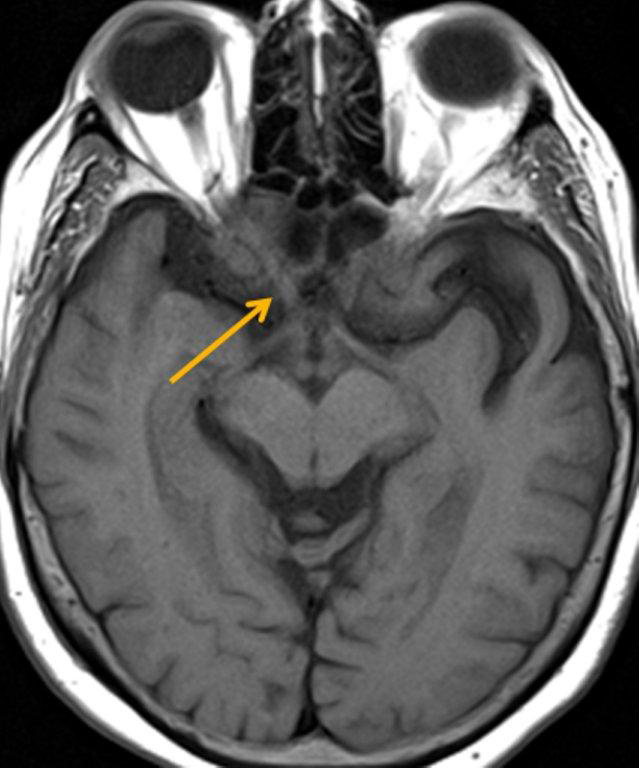
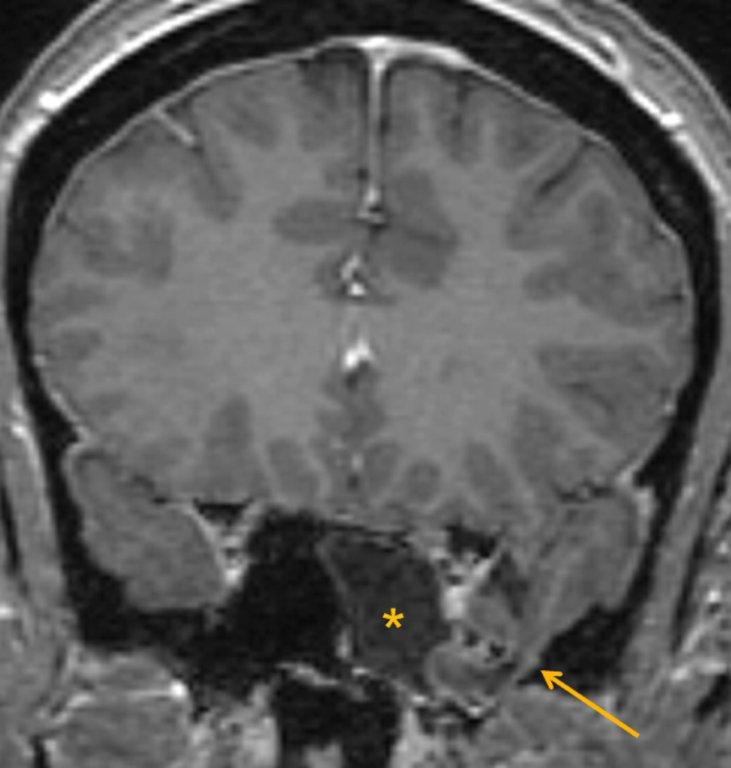
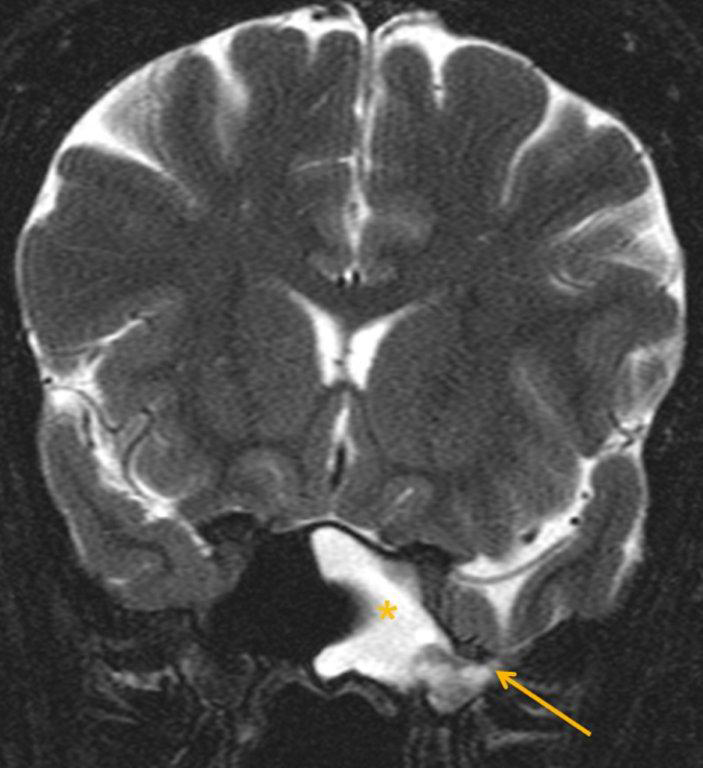
A cephalocele is a protrusion of intracranial contents through a defect in the calvarium or skull base; the defect may be congenital, acquired (including postoperative or post-traumatic) or spontaneous. The protrusion is termed a meningocele if it contains meninges and cerebrospinal fluid (CSF) and a meningoencephalocele or encephalocele when it also contains brain tissue. Regardless, a basal cephalocele may present as fluid or soft tissue in a paranasal sinus. Skull-base defects can also be a source of CSF leak and ascending infection without frank protrusion of extracranial contents.35 Frontal cephaloceles may present through a defect in the anterior cranial fossa into the ethmoid sinus or nasal cavity. Temporal cephaloceles may protrude into the sphenoid sinus through a defect in the sphenoid bone. Sphenoid cephaloceles have been divided into medial perisellar types and lateral sphenoid recess types.36 The lateral sphenoid cephalocele can be further divided into type 1 (which herniates into a pneumatized lateral recess, may simulate a retention cyst on CT, and may present with CSF leak and headache) and type 2 (which herniates into or through the greater wing of the sphenoid bone and may present with seizure or headache).37
CT and MRI are often complementary in the imaging of patients with CSF rhinorrhea.38 and sinus masses thought to represent an encephalocele. Isolated sphenoid sinus disease is unusual and one should at least consider the possibility of a cephalocele with a rounded soft tissue density in the sphenoid sinus. CT is excellent in demonstrating the bony skull base defect. MR better images the contents of the cephalocele, showing CSF and herniated meninges and/or brain tissue. CT cisternography can also be helpful in showing both demonstrating the bony defect and the communication of the subarachnoid space with the sinus contents (Figure 12).
Neoplasm
Malignant sinonasal neoplasms comprise 3% of all head and neck neoplasms. Squamous cell carcinoma accounts for 80% of sinonasal malignancy, arising most frequently in the maxillary sinus (25-63%), and less frequently in the nasal cavity (15-35%), ethmoid sinus (10-25%) and least frequently in the frontal and sphenoid sinuses (1%). Presenting symptoms of sinonasal malignancy can be identical to those caused by inflammatory sinus disease.39
A detailed description of the imaging of sinonasal malignancies is beyond the scope of this paper and the imaging features of the majority of tumors is nonspecific. Nevertheless, imaging is critical in distinguishing inflammatory sinus disease from neoplasm, characterizing the tumor and describing its extent. CT and MRI are complementary, with CT excelling in the assessment of bony changes and the evaluation of fibro-osseous lesions.
CT findings worrisome for malignancy include unilateral sinus disease, bony involvement, extensive soft tissue mass, tumor necrosis and lymphadenopathy (Figure 13).40 Bony changes may be seen in both inflammatory and malignant sinus disease. Erosion is highly suspicious for malignancy but can also be seen in inflammation. Erosions seen in chronic maxillary sinusitis are short, irregular and in the region of a normal dehiscence. Bone erosion of the infratemporal surface of the maxillary sinus is frequently seen with malignancy but not in chronic sinusitis; its presence strongly suggests neoplasm (Figure 14).41
MRI is superior in evaluating soft tissues (including differentiating tumor from trapped secretions) and aggressive lesions, assessing for intraorbital and intracranial extent, and identifying perineural tumor spread. As already mentioned, fungal disease and inspissated secretions may be a potential pitfall on MRI (Figure 15).27
Conclusion
The differential for an opacified paranasal sinus is broad. Knowledge of the etiologies discussed here and a systematic approach when reviewing images can be helpful in both narrowing the differential and formulating a correct diagnosis.
References
- Lev MH, Groblewski JC, Shortsleeve CM, Curtin HD. Imaging of the sinonasal cavities: inflammatory disease. Appl Radiol. 1998; 27(1): 20-30.
- Babbel RW, Harnsberger HR, Sonkens J, Hunt S. Recurring patterns of inflammatory sinonasal disease demonstrated on screening sinus CT. AJNR Am J Neuroradiol. 1992;13:903-912.
- Illner A, Davidson HC, Harnsberger HR, Hoffman J. The silent sinus syndrome: clinical and radiographic findings. AJR Am J Roentgenol. 2002;178:503-506.
- Levin SB, Mitra S. Maxillary sinus involution after endoscopic sinus surgery in a child: a case report. Am J Rhinol. 2000;14(1):7-11.
- Hobbs CG, Saunders MW, Potts MJ.Imploding antrum or silent sinus syndrome following nasotracheal intubation. Br J Ophthalmol. 2004;88:974-975.
- Rose GE, Sandy C, Hallberg L, Moseley I. Clinical and radiologic characteristics of the imploding antrum, or silent sinus, syndrome. Ophthalmology. 2003; 110: 811-818.
- Hourany R, Aygun N, Della Santina CC, Zinreich SJ. Silent sinus syndrome: an acquired condition. AJNR Am J Neuroradiol. 2005; 26: 2390-2392.
- Wise SK, Wojno TH, DelGaudio JM. Silent sinus syndrome: lack of orbital findings in early presentation. Am J Rhinol. 2007; 21(4): 489-494.
- Gill HS, Silkiss RZ. Diagnosis and management of silent sinus syndrome. EyeNet. 2011; 37-38.
- Shkoukani MA, Caughlin BP, Folbe A, et al. Mucoceles of the paranasal sinuses: a 10 year single institution review. J Otol Rhinol. 2013; 2(1): 1-3.
- Obeso S, Llorente JL, Rodrigo JP, et al. Paranasal sinuses mucoceles. Our experience in 72 patients. Acta Otorrinolaringol Esp. 2009; 60(5): 332-339.
- Klink T, Pahnke J, Hoppe F, Lieb W. Acute visual loss by an Onodi cell. Br J Ophthalmol. 2009; 801-802.
- Allmond L, Murr AH. Radiology quiz case 1. Arch Otolaryngol Head Neck Surg. 2002; 128: 596-599.
- Hesselink JR, Weber AL, New PF, et al. Evaluation of mucoceles of the paranasal sinuses with computed tomography. Radiology. 1979; 133: 397-400.
- Van Tassel P, Lee YY, Jing B, De Pena CA. Mucoceles of the paranasal sinuses: MR imaging with CT correlation. AJNR Am J Neuroradiol. 1989; 153: 407-412.
- Lanzieri CF, Shah M, Krass D, Lavertu P. Use of gadolinium-enhanced MR imaging for differentiating mucoceles from neoplasms in the paranasal sinuses. Radiology. 1991; 178: 425-428.
- Aribandi M, McCoy VA, Bazan III C. Imaging features of invasive and noninvasive fungal sinusitis: a review. Radiographics. 2007; 27: 1283-1296.
- Zinreich SJ, Kennedy DW, Malat J, et al. Fungal sinusitis: diagnosis with CT and MR imaging. Radiology. 1988; 169: 439-444.
- Dillon WP, Som PM, Fullerton GD. Hypoin-tense MR signal in chronically inspissated sinonasal secretions. Radiology. 1990;174: 73-78.
- Ustuner E, Fitoz S, Atasoy C, et al. Bilateral maxillary dentigerous cysts: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 95(5): 632-635.
- Han MH, Chang KH, Lee CH, et al. Cystic expansile masses of the maxilla: differential diagnosis with CT and MR. AJNR Am J Neuroradiol. 1995;16:333-338.
- Pierse JE, Stern A. Benign cysts and tumors of the paranasal sinuses. Oral Maxillofac Surg Clin North Am. 2012;24:249-264.
- Dunfee BL, Sakai O, Pistey R, Gohel A. Radiologic and pathologic characteristics of benign and malignant lesions of the mandible. Radiographics. 2006;26:1751-1768.
- Weissman JL, Snyderman CH, Yousem SA, Curtin HD. Ameloblastoma of the maxilla: CT and MR appearance. AJNR Am J Neuroradiol. 1993; 14:223-226.
- Towbin R, Dunbar JS, Bove K. Antrochoanal polyps. AJR Am J Roentgenol. 1979;132:27-31.
- Weissman JL, Tabor EK, Curtin HD. Sphenochoanal polyps: evaluation with CT and MR imaging. Radiology. 1991;178:145-148.
- Branstetter BF, Weissman JL. Role of MR and CT in the paranasal sinuses. Otolaryngol Clin North Am. 2005;38:1279-1299.
- Frosini P, Picarella G, De Campora E. Antrochoanal polyp: analysis of 200 cases. Acta Otorhinolaryngol Ital. 2009;29:21-26.
- Drutman J, Harnsberger HR, Babbel RW, et al. Sinonasal polyposis: investigation by direct coronal CT. Neuroradiology. 1994; 36(6): 469-472.
- Drutman J, Babbel RW, Harnsberger HR, et al. Sinonasal polyposis. Semin Ultrasound CT MR. 1991;12(6):561-574.
- Liang EY, Lam WW, Woo JK, et al. Another CT sign of sinonasal polyposis: truncation of the bony middle turbinate. Eur Radiol. 1996;6(4):553-556.
- Assanasen P, Naclerio RM. Medical and surgical management of nasal polyps. Curr Opin Otolaryngol Head Neck Surg. 2001;9:27-36.
- Cornelius RS, Martin J, Wippold FJ, et al. ACR appropriateness criteria sinonasal disease. J Am Coll Radiol. 2013;10:241-246.
- Yousem DM. Imaging of sinonasal inflammatory disease. Radiology. 1993;188:303-314.
- Quint DJ, Levy R, Cornett J, et al. Spontaneous CSF fistula through a congenitally fenestrated sphenoid bone. AJR Am J Roentgenol. 1996;166:952-954.
- Lai SY, Kennedy DW, Bolger WE. Sphenoid encephaloceles: disease management and identification of lesions within the lateral recess of the sphenoid sinus. Laryngoscope. 2002;112:1800-1805.
- Settecase F, Harnsberger HR, Michel MA, et al. Spontaneous lateral sphenoid cephaloceles: anatomic factors contributing to pathogenesis and proposed classification. AJNR Am J Neuroradiol. 2014;35:784-789.
- Mostafa BE, Khafagi A. Combined HRCT and MRI in the detection of CSF rhinorrhea. Skull Base. 2004;14(3):157-162.
- Madani G, Beale TJ, Lund VJ. Imaging of sinonasal tumors. Semin Ultrasound CT MR. 2009;30: 25-38.
- Madani G, Beale TJ. Differential diagnosis in sinonasal disease. Semin Ultrasound CT MR. 2009;30:39-45.
- Silver AJ, Baredes S, Bello JA, et al. The opacified maxillary sinus: CT findings in chronic sinusitis and malignant tumors. Radiology. 1987;163:205-210.