Technology and Industry: Ceiling-mounted MR, time-of-flight PET/CT, and other new products
Several new products have recently been released into the radiology marketplace. They include a ceiling-mounted magnetic resonance imaging (MRI) system, the first time-of-flight positron emission tomography/computed tomography (PET/CT) system, a new injector for vertebroplasty, and a real-time system for treatment planning in brachytherapy treatment of prostate cancer.
Siemens and IMRIS offer ceiling-mounted MRI
Siemens Medical Solutions (Malvern, PA) has signed a global original equipment manufacturer agreement with IMRIS, Inc. (Winnipeg, Manitoba, Canada) permitting the incorporation of Siemens' MAGNETOM Espree and MAGNETOM Symphony MRI systems into IMRIS' ceiling-mounted surgical imaging system, iMotion.
The iMotion MRI is mounted on the ceiling via a track system and can be moved between the diagnostic imaging area and the operating room (OR), allowing the system to be used in multiple locations. "The challenge of having an MRI system solely in the OR is that it can be costly and may take medical facilities years to recoup their investment," said David Graves, President, IMRIS. "By offering a system that can be used in the OR as well as the diagnostic imaging department, a facility can realize a significantly improved return on investment."
The MAGNETOM Espree, a 70-cm open-bore 1.5T MRI system, features Siemen's proprietary Tim (Total imaging matrix) technology. At 1.25 meters in length, it was designed to address issues related to obesity, claustrophobia, and elderly and pediatric patients, while capturing high-field 1.5T quality diagnostic images. Tim technology is based on a whole-body surface coil design that combines up to 102 integrated coil elements with up to 32 independent radiofrequency (RF) channels. The system also features Siemens' syngo applications, including syngo SPACE for 3-dimensional (3D) imaging with contrasts for complex spine, head, inner ear, abdomen, and pelvis cases; syngo SWI (susceptibility-weighted imaging) for visualization of bleeding, contusions, shearing injuries, and identification of minute intracranial malformations in stroke and brain trauma patients; and syngo GRAPPA for spine imaging in small children or patients with severe back pain.
Philips introduces time-of-flight PET/CT technology
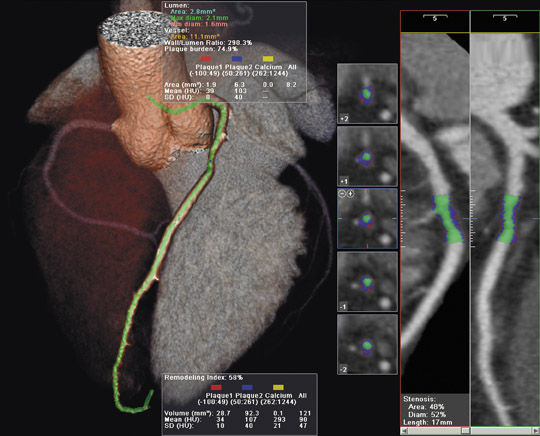
Philips Medical Systems (Bothell, WA) introduced its latest PET/CT system at the European Congress of Radiology meeting in Vienna, Austria in March 2006. The new system, known as the GEMINI TF, uses atomic particle time measurements to increase imaging sensitivity in the PET component.
According to the company, GEMINI TF, the first commercially available time-of-flight PET/CT system, more accurately tracks gamma rays using minute time measurements. This technology raises the effective image sensitivity by more than 2 times over conventional PET, the company notes. The system, which features the Philips' proprietary OpenView gantry design, can perform a whole-body PET scan in <10 minutes, even on larger patients who may have previously needed extended scan times (Figure). In addition to added patient comfort, the open gantry was designed to provide greater patient access for the administration of radiopharmaceuticals or for access to patient monitoring equipment.
In a conventional PET system, a decaying radioactive agent is injected into the patient. As each nucleus decays, it releases a positron that immediately collides with an electron, releasing 2 gamma rays that travel away from the collision zone at 180º from each other. These pairs are observed by the PET scanner, which uses the information to calculate where the agent is concentrated, thus creating an image of the affected area. This new system uses the company's proprietary TruFlight technology to measure the time difference in picoseconds (10 -12 seconds) between the arrival of the gamma rays in the pair. This enables the system to more accurately predict the point of origin of the gamma ray and, therefore, provide more accurate imaging.
The GEMINI TF, which received 510(k) clearance from the U.S. Food and Drug Administration (FDA) last November, is expected to become commercially available in the United States in the second quarter of 2006. The company plans to offer the system with either a 16- or 64-slice CT component, with a price range of approximately $2.7 million to $3.4 million.
Cardinal Health unveils new vertebral augmentation system
Cardinal Health (Dublin, OH) introduced a new vertebral augmentation system, the Vertebrex system, at the 31 st Annual Scientific Meeting of the Society of Interventional Radiology in Toronto, Ontario, Canada. This cement injection system, designed for the treatment of vertebral compression fractures, features a new design for the cement tube, an automatic mixing motor, and a closed system to reduce odor.
The injector barrel comes preloaded with radiopaque polymethylmethacrylate (PMMA) powder, which eliminates the need for measuring and pouring. The system also has a computerized motor that is designed to mix the cement inside the injector barrel and provide a more uniform cement consistency. The closed system design, with its enclosed glass monomer ampoule, reduces the odors associated with cement mixing and eliminates the sharps risk commonly associated with the use of glass ampoules.
A quick release feature on the injector provides rapid air purging and cement priming at the start of the procedure. It can also be used as a pressure release, if necessary. The cement tube extends through the access cannula as a liner that is primed prior to insertion, which allows cement to be injected into the vertebral body at the first turn of the system, reducing the risk of injecting a cement bolus. At the conclusion of the procedure, the cement cannula is removed before the access cannula, leaving a clean access cannula and reducing the risk of cement trailing out of the vertebral body.
Varian debuts real-time brachytherapy treatment planning system
Varian Medical Systems (Palo Alto, CA) recently introduced a real-time brachytherapy treatment planning system designed to speed up prostate cancer treatments and reduce the time patients spend in the hospital. The ultrasound-based treatment planning system, known as Vitesse 2.0, should allow clinicians to complete 2 brachytherapy procedures per day on a patient rather than spacing treatments over as many as 3 days.
With the new system, clinicians can develop treatment plans that show the locations of radiation sources and dose distribution, using ultrasound images generated in the operating room rather than CT. "Previously, hospitals wishing to use this technique have been hampered by the difficult logistics," explained William Hyatt, Varian's Vice president for Oncology Systems Operations and Brachytherapy Products. "The implant takes an hour, the patient goes to recovery, then goes to radiology for a CT scan, then waits for the physics staff to generate a treatment plan, and then gets the first radiation treatment. Often, by then, it is too late to get a second radiation dose on the same day and the patient must be kept in the hospital overnight. With the new system, the process is simply implantation, treatment planning during recovery, then treating. This can save up to 2 hours in the time it takes to get patients to their first treatment."
Clinicians can plan needle locations, monitor and adjust the positions as the needles are inserted, identify the final needle position in the patient, and export the dataset to the company's BrachyVision 3D planning system in real time.
