Stent grafts for abdominal and thoracic aortic disease
Images







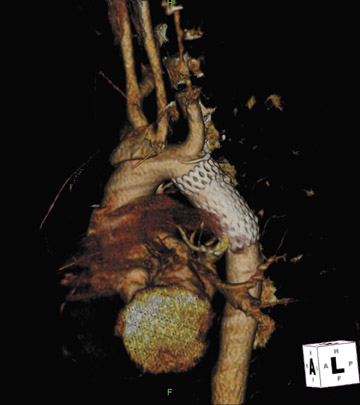
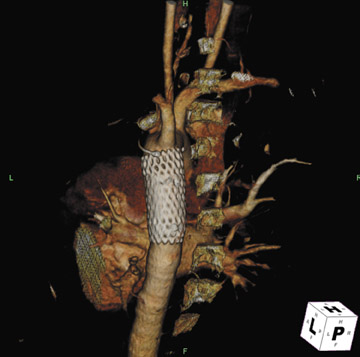
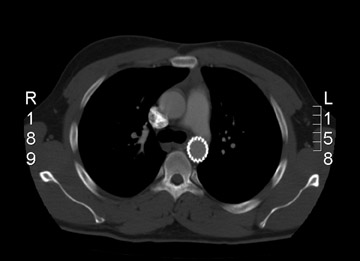
Dr. Kinney and Dr. Rivera-Sanfeliz are Associate Professors of Radiology, Department of Radiology, UCSD Medical Center, and Dr. Ferrara is Chief of Interventional Radiology, Naval Medical Center, San Diego, CA.
Transluminal placement of an endovascular stent was initially conceived by Charles Dotter. 1 Previous to this time, many efforts to treat aneurysms focused on wire embolization. The first successful resection and in situ bypass of an abdominal aortic aneurysm (AAA) was performed by Dubost in 1951. 2 Dr. Dotter's initial proposal was followed-up with feasibility research, using animal models of AAA. 3-6 From this initial pioneering work, stent grafts have evolved into a significant adjunct in the treatment of a variety of abdominal and thoracic aortic pathologic conditions that will be reviewed in this article.
Abdominal aortic aneurysm
An aneurysm is a focal dilation >1.5 times the adjacent normal caliber artery. Abdominal aortic aneurysms occur in 5% to 7% of people over the age of 60 in the United States. 7 It is estimated that 2.7 million Americans have AAA, but only half have been properly diagnosed. When the abdominal aorta is >3 cm in diameter, an AAA is diagnosed. Abdominal aortic aneurysm is the 13th leading cause of death in the United States. The infrarenal AAA is an arteriosclerotic aneurysm in 90% to 95% of patients, and in most cases, it remains asymptomatic until rupture occurs. Other causes of aneurysms include bacterial infection (mycotic), traumatic, or anastomotic pseudoaneurysms; a small set of AAAs are of the infiammatory type (<10%). Abdominal aortic aneurysms can rarely occur in juxta- and suprarenal locations. Abdominal aortic aneurysms predominate in men (85%), but AAAs tend to rupture in women at an overall smaller diameter than in men. The indication for therapy of AAA varies, but when the diameter is >5 cm (some advocate 4 cm, the so-called "small AAA") open surgical repair is considered. At this diameter, the risk of death from rupture over 1 year exceeds the risk of the operative repair. Most experts agree that the annual risk of rupture for aneurysms ≥6 cm is at least 10% per year, while with aneurysms >7.5 cm the risk may be as high as 30%! The particular risk varies with individual patients, however, since some patients have higher operative comorbidities. Abdominal aortic aneurysm may also be considered for repair at smaller sizes if they are painful, have caused distal embolization, or are rapidly enlarging (>0.5 cm/year).
Conventional AAA therapy constitutes major surgery requiring postoperative intensive care unit (ICU) monitoring, a 5- to 7-day hospitalization, and 6 to 8 weeks of overall recovery. Open repairs typically use general anesthesia. The exposed aorta is clamped beneath the renal arteries, the AAA sac is opened, the thrombus is removed, and side branches are controlled surgically. A Dacron graft (DuPont, Wilmington, DE) is sutured in place, and the native AAA sac closed over the graft. Short AAA necks may require suprarenal clamping, which entails added risks. Procedural risks of AAA repair include: Pulmonary, cardiac, and renal failure; postoperative ileus; embolization into renal, mesenteric, and lower extremities; infection; blood loss; paralysis or other nerve injury (including sexual dysfunction); and colonic ischemia. In large contemporary series, the hospital mortality after elective aneurysm repair ranges from 2.8% to 6.2%; coronary artery disease is the major risk factor for postoperative death. For ruptured AAAs, the hospital mortality is as high as 60%. Currently, approximately 15,000 aneurysm ruptures with fatal outcomes are documented in the United States annually. Approximately 40,000 patients undergo aneurysmorrhaphy each year. Despite improved screening methods, approximately 20% to 30% of all AAA repairs still occur emergently.
Aneurysm imaging
Image-guided diagnosis has contributed greatly to the impressive success of modern vascular surgery for AAAs. A wide array of imaging systems can be used in the diagnosis and characterization of AAAs (Table 1).
Selection of the optimal AAA diagnostic imaging modality is determined by the clinical symptoms, patient status, and availability of equipment. In unstable patients, operative repair may be done without imaging. In stable suspected rupture cases, imaging findings facilitate surgical planning. Routine screening is performed easily with ultrasound studies; although, presently, computed tomography (CT) appears to offer many advantages. Angiography is reserved for problem solving, such as determination of accessory renal arteries, defining the AAA neck, evaluating for side-branch disease, and delineating runoff status in claudicators.
Because ultrasound is quick, inexpensive, and accurate, and elective surgery has an acceptably low mortality, some researchers have advocated screening for AAA. 8 The 12-fold increase in operative deaths for ruptured as compared with nonruptured AAA clearly suggests that more emphasis needs to be placed in identifying and repairing asymptomatic lesions.
With the advent of endovascular repair of AAA, more detailed analysis of aneurysm anatomy is required to properly plan the procedure than is required for conventional repair (Table 2). This information is readily obtained with a spiral CT technique, with supplemental information obtained with catheter angiography and occasionally with endovascular ultrasound.
Commercially available endografts for AAA
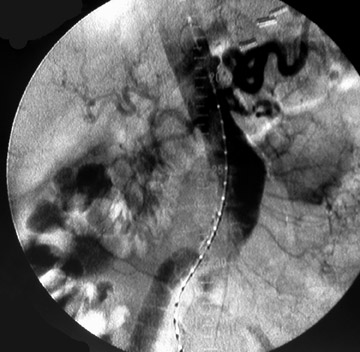
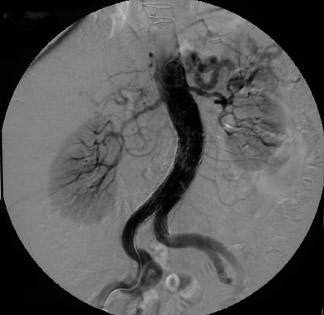
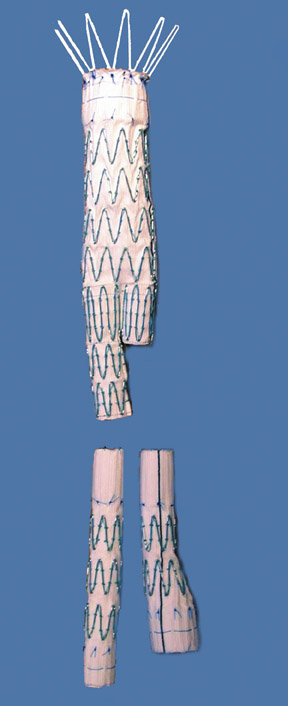
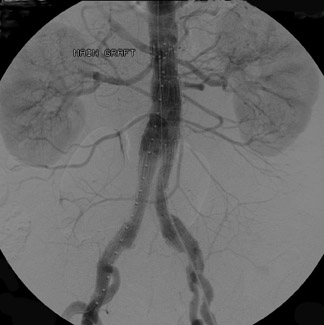
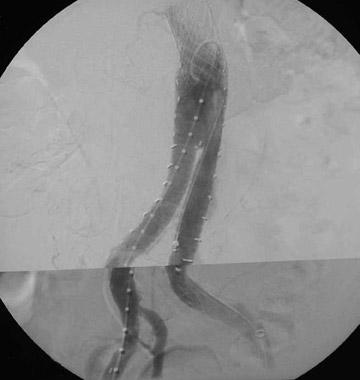
Presently, 4 endografts have been approved by the Food and Drug Administration (FDA) for repair of AAA, but only 3 are currently commercially available. Ancure (Guidant Corp., Indianapolis, IN), the first approved device, was removed from the market on October 1, 2003. The other endovascular devices are the AneuRx (Medtronic, Inc., Santa Rosa, CA), the Excluder (W. L. Gore & Associates, Flagstaff, AZ), and the Zenith (Cook, Inc., Bloomington, IN) (Table 3; Figures 1 through 3).
Implantation of AAA stent grafts
Stent-graft design has evolved over the past decade. The ideal stent graft remains an elusive goal, but successive iterations have improved deployment characteristics, reduced profiles, and improved durability. In general terms, stent grafts can be classified in a number of ways: 1) unibody construction (tubes, bifurcated designs, and aorto-uni-iliac versions) versus modular; 2) supported versus unsupported endografts; 3) hook fixation versus friction fit; and 4) infrarenal versus transrenal fixation. Considering all clinical and morphologic prerequisites, it is estimated that only 20% to 30% of all patients with infrarenal AAA are candidates for endovascular repair. The technical success of stent-graft insertion has progressively risen. Improvements in technique, patient selection, and preoperative imaging and planning have all played a role, but the main factor appears to be improved device performance. 9 The technical details describing insertion of each particular graft exceeds the scope of this article.
Imaging follow-up of stent-graft devices
All patients who undergo stent-grafting have to be informed of the importance of follow-up imaging, as long-term complications may occur that may result in the patient not being adequately protected. The various manufacturers have slightly different follow-up schedules (Table 4). 10 Follow-up imaging repeatedly assesses the aneurysm size, detects endoleaks, and monitors the structural and positional integrity of the stent graft.
Endoleaks
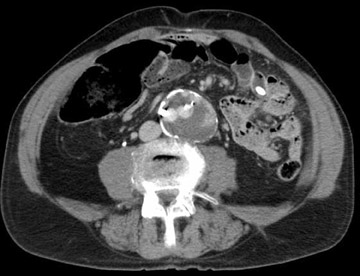
Endoleak refers to persistence of fiow within the aneurysm sac despite an endovascular prosthesis, and these are classified by the cause of the leak (Table 5). 11 The significance of endoleak is controversial and hotly debated. Endoleaks can be detected with several imaging modalities. 10 Triphasic CTA is the most commonly used modality to follow-up endovascular repair of AAAs. Noncontrast CT allows assessment of migration by careful evaluation of the aortic and iliac deployment sites on serial studies. These also detect calcification that may mimic leaks. The arterial phase appraises patency of lumens and side branches. The delayed postcontrast phase detects leaks. Ultrasound is used in patients who are unable to receive contrast. Catheter angiography is helpful to investigate leaks detected by other modalities or to study suspected leaks in patients whose AAAs do not shrink despite endoprosthesis placement. These are tailored examinations based upon the problem at hand. In general, each anastomosis is studied along with injections of the superior mesenteric artery (SMA), renals, and internal and external iliacs. Magnetic resonance angiography can also be used for endoleak detection; some suggest this method has added sensitivity. 12
With CT angiograms, careful side-by-side comparisons are imperative for accurate AAA sac size measurements. Review of preplacement CT images may detect interval AAA growth not noticed on sequential follow-up CTAs. If the AAA is growing and the endoleak is identified on CT, transcatheter correction is warranted. If the AAA sac is growing, but a leak is not seen with CTA, angiography with careful attention to anastomotic connections and side branches is performed.
Endoleak management
Type I endoleak
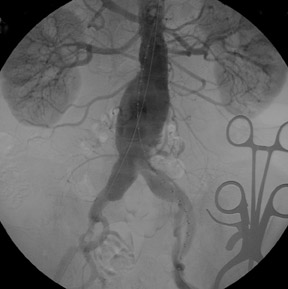
With type I leaks, there is direct communication between the lumen and the AAA sac and the patient is not protected from AAA rupture; these leaks are typically detected during the procedure and repaired immediately (Figure 4). If patient selection and preprocedural planning is done carefully, the need to treat type I endoleaks may be avoided. All measurements must be done carefully; it is best to err on the side of oversizing the device diameters to help with seals. Aortic attributes believed to infiuence these leaks include neck diameter, length, and angulation. Infiation of a balloon across the anastomosis with a type I leak may seal the leak. Addition of aortic cuffs or iliac extenders may solve leaks if additional graft material is required. If there is insufficient room for a covered stent graft, placement of a noncovered stent may affect a seal. If these maneuvers are ineffective, consideration of open conversion becomes necessary. Proximal type I leaks may develop later if the device migrates inferiorly, and these may be treated with addition of aortic cuffs.
Type II endoleak
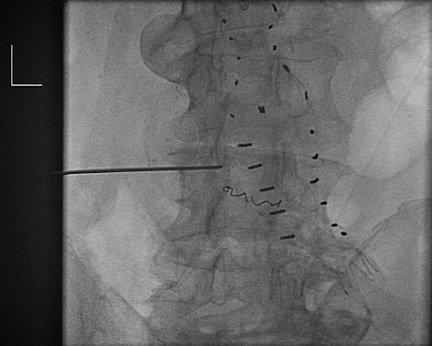
Management of these leaks is most controversial because one third to one half of these eventually thrombose, typically within the first 6 to 12 months. Apprehension occurs because direct AAA sac pressure measurements have documented near systemic blood pressures in some cases. 13 In a sac with a type II endoleak that is shrinking or stable, conservative management is utilized, with follow-up scans at 3- to 6-month intervals. The follow-up interval depends upon the size of the leak, the size of the feeding vessel, and the overall AAA size. A smaller aneurysm should tolerate more growth before rupture than would a larger AAA. If treatment is considered (ie, if the sac has progressively increased), transcatheter embolization can be tried (Figure 5). Type II leaks require infiow and an outfiow vessel (or outfiow vessels) for patency. Treatment of the infiow or outfiow artery and the sac may be needed for closure. Microcatheters allow for precise delivery of coils and gelfoam. Injection of thrombin has been associated with neurologic impairment that is possibly related to embolization of spinal arteries or the lumbar plexus. Other investigators have attempted to enter the sac by puncturing the seal at an anastomosis (proximal or distal) and negotiating a catheter into the sac. Direct puncture may be done with CT, sonographic, or fiuoroscopic guidance. This allows embolization of the endoleak with coils or glue (n-butyl-cyano-acrylate; TRU-FILL, Cordis Neurovascular, Inc., Miami Lakes, FL).
Type III endoleaks
Like type I endoleaks, type III endoleaks are dangerous and require treatment. Proper overlap of modular components minimizes the occurrence of these leaks. Balloon dilation of the leaking segments may seal these as well. If the balloon is too large, tears in the graft fabric may result in type III leaks. Placement of cuffs and extenders may seal these.
Type IV endoleaks
Porosity of graft material may result in an impressive leak at the time of graft placement. The early AneuRx grafts all demonstrated this leak at angiography and CT scanning within 24 hours of insertion. The WALLGRAFT (Boston Scientific, Natick, MA) may also show this type of leak. One must be aware of this property of the grafts being used, as this leak is self-limited over a few days.
Type V endoleaks
Open conversion is apparently required for these cases, as the sac remains pressurized and no cause for the leak is identified. It appears that increased pressure within the AAA sac (endotension) can be transmitted through clot.
Results of endovascular stent grafts for AAA
Endovascular AAA repair has gained acceptance as a minimally invasive alternative to open surgery in selected patients. While long-term durability remains a major concern, patients and their physicians are willing to accept a degree of uncertainty in exchange for dramatic reductions in hospital stays, need for blood transfusions, and postoperative recovery times. 14 This is clearly illustrated by a comparison of 5-year data of the Guidant Ancure bifurcated AAA stent graft with open repair. 15 No aneurysm ruptures were reported in endovascular patients followed for 5 years. (Note that ruptures did occur in patients treated with the first-generation Ancure tube [nonbifurcated] graft). The 30-day morbidity was 28.8% and 44.4% in the endovascular and open arms, respectively. Patients in the endovascular arm were more likely to have arterial trauma and hematoma, while the surgical-arm patients were more likely to have bleeding, and bowel, cardiac, and respiratory problems. The 30-day mortality was 1.7% versus 2.7% (not significant [NS]) in the endovascular and open arms, respectively. The immediate benefits of endovascular repair compared with open repair include shorter hospital stay (2 versus 6 days), decreased blood loss (400 versus 800 mL), and reduced ICU use (33% versus 94%). Only 9 patients (2.8%) required late open conversion. These patients were distributed over the 5-year period, which emphasized the necessity of follow-up. Long-term survival rates were similar.
Various endovascular devices have been evaluated in clinical trials designed to gain FDA approval. The devices and studies differ in many regards, so interdevice comparisons are difficult. One study recently published by the Cleveland Clinic summarizes many issues with endovascular therapy of AAA. 16 Over a 6-year period, 703 patients underwent endovascular repair of infrarenal AAA. Patient ages ranged from 48 to 100 years with an average of 75 years. Five devices were used in the study: Ancure (n = 63), AneuRx (n = 203), Excluder (n = 25), Talent (n = 39), and Zenith (single center [n = 181] and multicenter data [n = 144]). The operative 30-day mortality was 1.7% (12 patients). Elective AAA repair had a 30-day mortality of 1.1%, while "urgent AAA repairs" had a mortality of 19%. Patient survival with stent-grafting was 90% at 1 year, 78% at 2 years, and 49% at 5 years; survival did not vary with the device used. Aneurysm-related death occurred in 12 patients (1.7%) with no device-specific differences. Three aneurysms ruptured after device implantation, at 4, 7, and 19 months, for a rupture-free probability of 98.7% ± 0.9% at 24 months. Secondary procedures were required in 104 patients (15%). The 12-month risk for secondary intervention did not differ between device groups ( P = 0.333), ranging from 8.8% ± 2.1% in the AneuRx to 20% ± 5.6% in the Zenith (multicenter trial). Graft limb occlusion was more common with the Ancure device (unsupported) (11% ± 4.6% at 12 months). Late limb occlusion was rare. The 12-month risk for migration ranged from 0% (Ancure, Excluder, and Talent) to 8.2% ± 4.3% (Zenith), but the differences were not statistically significant. Endoleak of any type was documented in 162 patients (23%). Kaplan Meier risk for endoleak was 22% ± 1.9% at 6 months, 30% ± 2.3% at 12 months, and 42% ± 3.4% at 24 months. Type I leak was found in 21 patients (3%), type II in 130 patients (18%), and type III in 16 patients (2.3%). Interestingly, type I and III leaks were not infiuenced by stent-graft design, although type II leaks were (Excluder 58% at 12 months and 19% in the Talent). Aneurysm shrinkage (diameter reduction >5 mm) was the following: 6 months (8.3% ± 1.4%), 12 months (39% ± 2.7%), 24 months (60% ± 3.1%), and 36 months (68% ± 3.6%). Aneurysm sac enlargement was the following: 6 months (1.8% ± 0.7%), 12 months (3.5% ± 1.0%), 24 months (11% ± 2.5%), and 36 months (21 ± 4.5%).
Thoracic applications
The incidence of thoracic aortic aneurysms (TAAs) and acute and chronic type B dissections is estimated to be as high as 10 cases per 100,000 people per year. 17 The natural history of untreated disease includes progressive enlargement, increasing risk of rupture, and, ultimately, death, with 2-year survival rates of <30%. Traditional treatment involves graft replacement via left thoracotomy, which improves survival in comparison with medical therapy. Despite dramatic improvements in the technical expertise for performing these complex thoracic aortic surgeries, open surgery is complicated by operative mortality rates ranging from 8% to 20% for elective cases and up to 60% for emergencies. Survivors of open repairs still suffer from morbidity rates of up to 50% related to renal, intestinal, and spinal cord ischemia that substantially limit functional recovery.
The success of endovascular stent grafts for AAAs has provided motivation to adapt similar technology for descending TAAs and type B dissections. The preliminary results of such therapy appear promising, with advantages of the endografts including shorter operative time, avoidance of cardiopulmonary bypass, decreased need for general anesthesia, lack of aortic cross-clamp time, and avoidance of major thoracic or thoracoabdominal incisions. Most reported series document high technical success along with major reductions in morbidity and mortality. The FDA has recently granted conditional approval of the Gore TAG thoracic endoprosthesis for TAAs (W. L. Gore & Associates).
Thoracic aneurysms
Since 1992, there have been at least 13 reported series of patients undergoing endovascular repair of TAAs. Patient populations and devices used varied, but the results have been fairly impressive (Table 6). The Stanford Group reported 1- and 2-year survival rates of 81% and 73%, respectively. 18 This compares favorably with the results of traditional open surgery, where the actuarial survival rates are estimated to be 70% at 5 years, and 40% at 10 years in "survivors of open repair." These comparisons are difficult since most of the patients offered endovascular therapy have been denied surgery.
Acute and chronic aortic dissections
The endovascular approach has been applied to other thoracic aortic pathologies, including acute and chronic dissections, penetrating ulcers, and traumatic transections. Current indications for open repair of type B dissections include uncontrollable hypertension, persistent pain, expanding aneurysm, or end-organ ischemia. Acute dissection results in 36% to 72% mortality within 48 hours if left untreated; even operative therapy is associated with 50% to 70% mortality. With such dismal results, it is no wonder that endovascular approaches have been applied, and early, small series report lower mortality rates of 16% to 28%. 19 Stent-graft coverage of the proximal entry site of a type B dissection should, in theory, limit the extent of the dissection, obliterate the fiow into the entry tear, and promote thrombosis of the false lumen.
Traumatic transections and postoperative pseudoaneurysms
A small number of patients have had stent grafts used, with very encouraging results, to treat posttraumatic aortic transections and for postoperative pseudoaneurysms (Figure 6). 20-22 Those patients who survive aortic transaction are at particularly high risk of dying from further hemorrhage within the first 24 hours. If endovascular repair is going to play a major role in such cases, there is a need for the devices to be of sufficient caliber to seal the thoracic aorta and the ability to place such devices rapidly. Moreover, the long-term performance characteristics need to be better delineated before use in younger trauma patients is undertaken. Surgical repair of chronic traumatic aneurysms has been reported to have a mortality rate of 5% to 18%, a morbidity of 11% to 50% (related to bleeding, heart failure, renal failure, spinal cord injury), and a 53% incidence of Horner syndrome or vocalcord palsy. Stent grafts may potentially ameliorate these complications.
Conclusion
The development of endovascular repair of AAA has now entered its second decade with introduction of a multiplicity of designs (>16) with only 4 devices gaining FDA approval. 23 There has certainly been great progress in the design of endovascular devices, and several newer designs have completed, or are undergoing, evaluation. Up to this point, a mortality advantage for endovascular repair of AAA has not been convincingly shown compared with results of open repair, although recent trials are encouraging. Essentially, all trials thus far have shown significant reductions in systemic complications (cardiac and pulmonary) using the endovascular approach as opposed to open repair. Compared with conventional surgery, endovascular repair has shown reduced blood losses and transfusion requirements and reduced hospital and ICU stays with more rapid return to preoperative levels of function. Concerns about the durability of endovascular repair still remain. Endoleaks and limb thromboses continue to plague endovascular grafts, necessitating secondary interventions. The annual rate of AAA rupture after endovascular repair is approximately 1%. Performing endovascular grafts in AAA patients who are fit for open repair and have otherwise reasonable life expectancy seems unwise at this point. Endovascular repair of AAAs is appropriate in patients with significant comorbidities and suitable anatomy and in patients with relatively limited life expectancy and larger or expanding AAAs. Technical advances in design will allow expansion of the use of endovascular repairs for AAAs, in ruptured AAAs, and in other thoracic aortic conditions.