Radiological Case: Shoulder injury related to vaccine administration
Images
CASE SUMMARY
A 55-year-old woman presented with a 3-month history of left shoulder pain occurring less than 24 hrs after receiving an influenza vaccination and a tetanus, diphtheria, and pertussis (Tdap) vaccination. She described her pain as dull/achy, 5 out of 10 (10-point scale with 10 being the worst pain) at rest, and exacerbating with shoulder manipulation, most notably with abduction. In addition, she reported persistent 3-4/10 dull pain at rest, sleep disturbances due to pain, and difficulty with lifting, carrying and driving. The patient denied prior shoulder pain, fever, redness, soft tissue swelling, interval trauma, excessive lifting, pulling or exercising. Physical exam findings were positive for left deltoid tenderness, restricted adduction, and tenderness upon passive abduction and arm elevation—reflexes, strength, and sensation were intact. Serum C-reactive protein was normal.
After minimal avail from multiple sessions of physical therapy and oral pain medications, a non-enhanced MRI of the left shoulder was ordered.
IMAGING FINDINGS
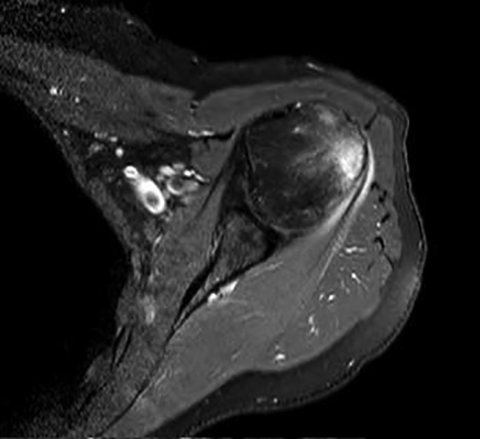
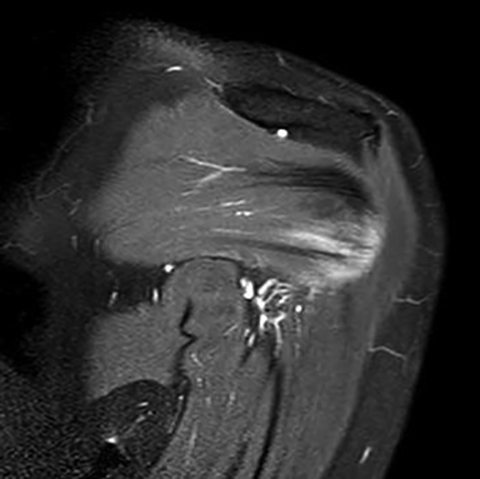
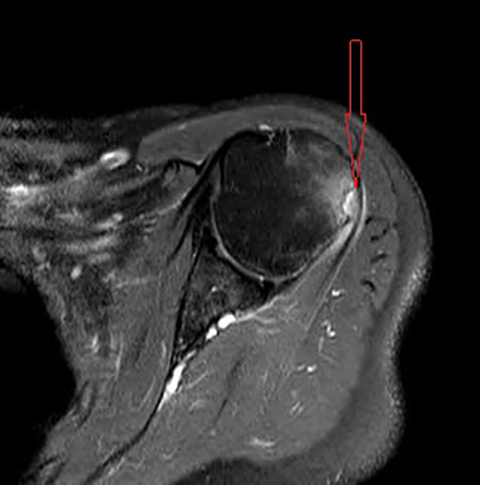
Initial non-enhanced MRI of the left shoulder demonstrated abnormal osseous edema in the lateral humeral head and neck, and abnormal soft tissue edema in the teres minor muscle, including its myotendinous junction and humeral insertion (Figures 1 and 2). These post-injection findings were attributed to sequela from direct needle impact and reactive change related to the injected material. A 3-month follow-up (after an ineffective subacromial corticosteroid injection) non-enhanced MRI demonstrated ongoing inflammatory disease as evidenced by new visualization of an 8-mm humeral head cortical erosion (Figure 3).
The patient’s pain persists, but at approximately 6 weeks post-injection, her pain with sleep reduced from 4/10 to 2/10. In addition, her rest pain reduced from 5/10 to 3/10 at approximately 7 weeks but with persistent mild restricted motion on physical exam.
DIAGNOSIS
Shoulder injury related to vaccine administration (SIRVA). Less likely differential considerations include focal septic arthritis, calcific tendinitis with intraosseous penetration, gout, and seronegative spondyloarthropathic enthesopathy.
DISCUSSION
Shoulder injury related to vaccine administration (SIRVA) is a sequela of the body’s immune response to direct intracapsular inoculation. Given the available literature on SIRVA, our patient’s presentation fits well within the described typical history and symptomatology—absence of prior shoulder dysfunction, rapid onset of pain, and limited range of motion.1 Assuming compliance with the current Centers for Disease Control and Prevention (CDC) vaccine administration protocol for a woman weighing less than 70 kg (our patient weighed 63 kg with a BMI of 22), a 1-inch needle was used for our patient’s injection. Using MR and CT to measure the deltoid fat pad and muscle thickness, Lippert and Wall studied the rate of over-penetration using the CDC-recommended needle lengths in pediatric patients of varying weights and BMI’s ranging in 3-18 years of age. They reported a risk ranging from 11-61% using the needle lengths recommended for each age group. Corroborating with Bodor and Montalvo’s sonographic deltoid study, which reported that the sub-deltoid bursa extends 3.0 to 6.0 cm (1.18 – 2.38 in) laterally beyond the acromion border and is 0.8 to 1.6 cm deep to the skin surface, Lippert and Wall made a weight-based recommendation that a ½ needle length should be used in any female weighing less than 70 kg, avoiding the top third of the deltoid to ensure a 0% risk of over penetration.2,3,6
Careful study of our patient’s chart noted that her symptoms began to improve, albeit minimally, around 6 weeks. This corresponds to a rabbit study by Dumonde demonstrating that antigen injected within synovial tissues was bound to existing antibody within the synovial tissues which resulted in a prolonged immune response lasting 6 weeks. As with most Americans of our patient’s age, she has received prior Tdap and influenza vaccinations. With that said, our patient’s prolonged response (in comparison to relatively brief discomfort resulting from a truly intramuscular injection) is likely due in part to preformed antibody existing within her synovial tissue from prior vaccinations.1,4,5
The humeral greater tuberosity erosion depicted on our patient’s follow-up MRI reflects not only sequela of prolonged immune response, but it also strongly suggests direct needle impact—likely secondary to the CDC-recommended needle length given our patient’s weight. One of the 13 patients in the 2010 case series by Atanasoff et al underwent arthroscopic shoulder repair during which the surgeons explored the deltoid needle trajectory (as identified by the patient). The needle path revealed inflamed and scarred bursa and thickened tissue around a damaged tendon. In addition, the path led to abnormally friable bone on the humeral greater tuberosity, which was noted to give way with pressure from the surgeon’s needle. Our patient did not undergo surgical repair, but her MR findings are strikingly suggestive of similar pathogenesis.
In addition to the persistent physical discomfort, diagnosing and managing SIRVA can be expensive. An uncomplicated flu shot is often free of charge; however, our patient’s initial “free” flu shot resulted in more than $18,000 of healthcare charges, not including the follow-up MRI and office appointments with her orthopedic surgeon. Fortunately, our patient has insurance and had to only pay about 20% of the cost out-of-pocket, unlike thousands of uninsured Americans receiving yearly vaccinations at local pharmacies and clinics nationwide.
CONCLUSION
Prolonged shoulder pain occurring acutely after vaccination should prompt physicians to incorporate SIRVA in their differential of possible causes. In addition, vaccine administrators in healthcare facilities nationwide should be educated on the potential risks (physical and economic) of over-penetration associated with the current CDC’s needle length recommendations.
REFERENCES
- Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010; 28(51): 8049-8052.
- Barnes M, Ledford C, Hogan K. A “needling” problem: Shoulder injury related to vaccine administration. J Am Board Fam Med.2012: 25(6): 919-922.
- Lipper WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008; 22(3): e556 – e563.
- Dumonde DC, Glynn LE. The production of arthritis in rabbits by an immunological reaction to fibrin. Brit J Exp Pathol. 1962;43: 373.
- Cooke TD, Jasin HE. The pathogenesis of chronic inflammation in experimental antigen-induced arthritis. I. The role of antigen on the local immune response. Arthritis Rheum. 1972;15(4): 327.
- Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007:25(4): 8049 – 8052.
Citation
Radiological Case: Shoulder injury related to vaccine administration. Appl Radiol.
December 17, 2014