Radiological Case: Autoimmune pancreatitis with renal involvement
CASE SUMMARY
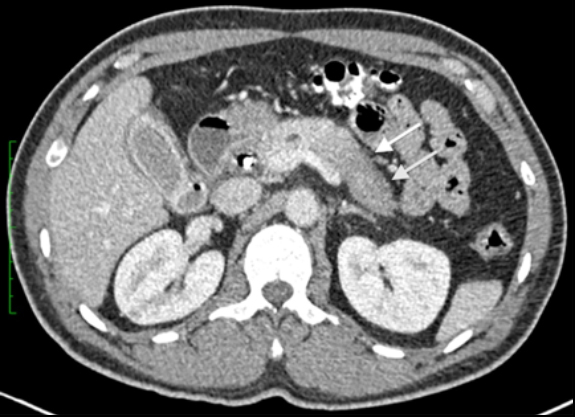
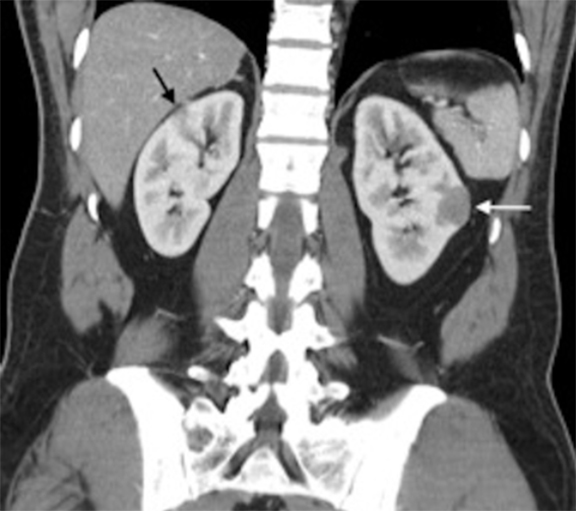
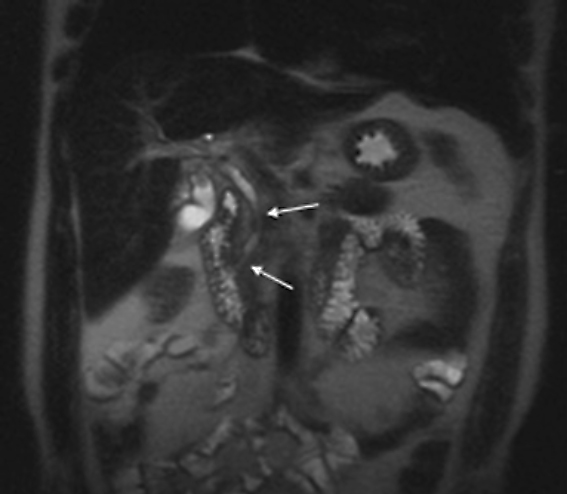
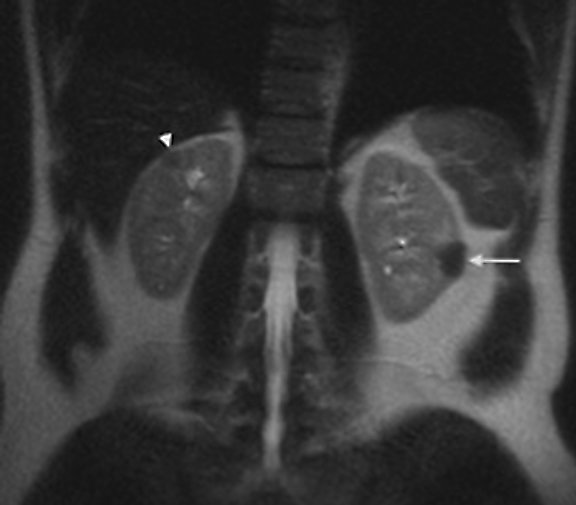
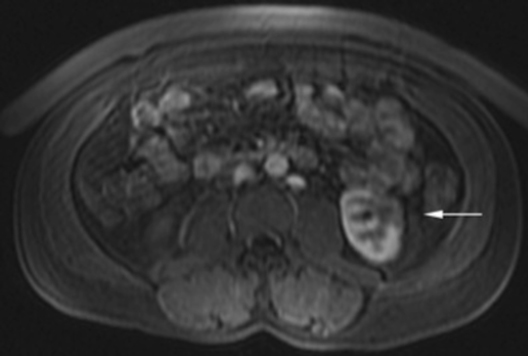
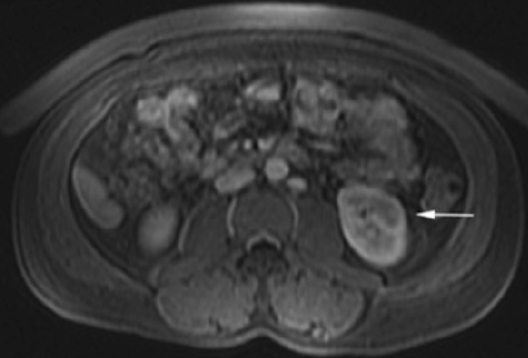
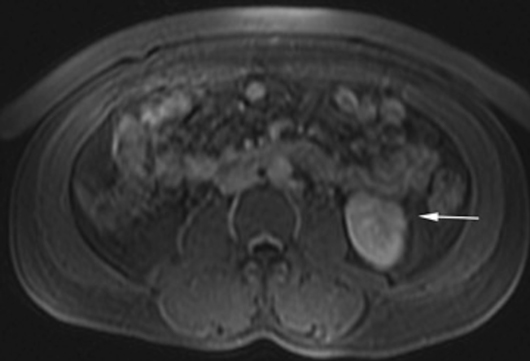
The patient was a 46-year-old male who was admitted to Harbor-UCLA Medical Center after presenting with a 2-month history of abdominal pain, nausea, vomiting and new-onset jaundice. An abdominal ultrasound was performed, revealing gallstones, a dilated common bile duct and a 2.4-cm hypoechoic left renal mass. Labs were significant for hyperbilirubinemia, transaminitis and elevated alkaline phosphatase. A CT of the abdomen and pelvis with contrast demonstrated a mildly enlarged pancreas with hypoenhancement of the pancreatic body and tail (Figure 1, click here to view DICOM images courtesy of Viztek), a dilated common bile duct and bilateral hypoattenuating renal masses (Figure 2). Tumor markers, including CEA, CA 19-9, CA-125 and chromogranin A, were within normal limits. An endoscopic retrograde cholangiopancreatogram (ERCP) was performed that demonstrated 2 distal CBD strictures and a biliary stent was placed. Intraductal biopsies obtained during the ERCP were negative for the presence of tumor cells. An abdominal MRI with contrast was performed that again showed CBD strictures (Figure 3), relative hypoenhancement of the pancreatic body and tail, and bilateral renal masses with relative hypoenhancement (Figures 4 and 5, click here to view DICOM images courtesy of Viztek). Eventually, the larger mass in the lower pole of the left kidney was biopsied under CT guidance. Histologic and cytologic analysis of the specimen demonstrated marked infiltration by IgG4 positive plasma cells and an increased number of lymphoid cells, including eosinophils, confirming the diagnosis of an AIP-associated renal mass.
IMAGING FINDINGS
Typically, autoimmune pancreatitis (AIP) involves the entire pancreas and manifests as a diffusely enlarged, sausage-like gland with decreased attenuation on CT.1-2 Pancreatic involvement may also be focal, typically involving the pancreatic head, or multifocal. When involvement is focal or multifocal, the involved segments tend to be well demarcated and can lead to upstream pancreatic duct dilatation, which may mimic pancreatic malignancy. The imaging findings associated with AIP include: hypoechogenicity on ultrasound (US), decreased attenuation on CT, mild hyperintensity on T2-weighted magnetic resonance (MR) imaging and hypointensity on T1-weighted MR imaging.3 Furthermore, with contrast-enhanced CT and MR imaging, decreased early phase enhancement followed by moderate delayed enhancement in the late phase is typically seen. A hypoattenuating or hypointense rim or “halo” is also commonly seen and believed to represent adjacent fluid, phlegmon or fibrous tissue.3, 4-5
The imaging characteristics of AIP-associated renal lesions were described in a study with 14 patients in 2007 by Takahashi et al. and again in 2010 by Triantopoulou et al. in a study involving seven patients. They found that renal involvement was predominantly bilateral and multiple, involved the cortex, and could appear in a number of ways that include: small cortical peripheral nodules, round lesions, well-defined wedge-shaped lesions or diffuse patchy involvement. The studies reported that the lesions displayed low attenuation on contrast-enhanced CT imaging and were not visible on non-contrast CT imaging. Lesions were isointense on T1-weighted imaging and hypointense on T2-weighted imaging. On gadolinium-enhanced MR imaging, the lesions were isointense during the early phase and had a tendency to become less distinct during later phases.6-7
DIAGNOSIS
Autoimmune pancreatitis with associated renal lesions. Differential diagnoses: pancreatic malignancy, acute pancreatitis, renal cell carcinoma.
DISCUSSION
AIP was first described in 1961 by Sarles et al as pancreatitis with hypergammaglobulinemia.8 In 2001, Hamano et al reported that autoimmune pancreatitis was closely associated with elevated serum levels of IgG4 and periductal pancreatic infiltration of IgG4-positive plasma cells. The infiltration by IgG4 positive plasma cells leads to periductal and interlobular fibrosis that ultimately results in narrowing of the pancreatic duct and acinar atrophy3. Subsequent research has suggested that AIP is part of an IgG4-related systemic disease process, an entity that was first described by Kamisawa et al. in 2003.9 AIP is the most common manifestation of IgG4-related disease, but extrapancreatic involvement is not uncommon, with up to 77% of cases having extrapancreatic involvement.3
In addition to the pancreas, other sites of involvement include the biliary system, kidneys, gallbladder, salivary glands, thyroid gland, retroperitoneum, liver and lung.6-7,10 The histologic features are the same for all organs involved and include: dense lymphoplasmacytic infiltration rich in IgG4-positive plasma cells and eosinophils, obliterative phlebitis and storiform fibrosis.2
Concerning renal involvement in AIP, four recent publications have reported a prevalence that ranges from 14-39%.2,6,11-12 Evaluation of several AIP associated renal lesions by Takahashi et al. and Triantopoulou et al. showed that the lesions displayed low attenuation on contrast-enhanced CT imaging, isointensity on T1-weighted MR imaging and hypointensity on T2-weighted MR imaging. Their studies also showed that on gadolinium-enhanced MR imaging, the lesions were isointense during the early phase and had a tendency to become less distinct during later phases.6-7 The masses present in our case were concordant with the typical published findings demonstrating T2 hypointensity and T1 isointensity. While the right renal masses were not well visualized on the contrast enhanced MRI, the mass within the lower pole of the left kidney demonstrated relative hypoenhancement in the early phases with gradual loss of distinct margins on later phases. The mass within the mid left kidney demonstrated relative peripheral hypoenhancement with a central region of nonenhancement, which differs from the typically seen pattern.
Distinguishing AIP from pancreatic malignancy or other forms of pancreatitis can be clinically challenging. This is especially true when pancreatic involvement is focal, which can further raise concern for malignancy. Several groups have proposed diagnostic criteria for the diagnosis of AIP. Most recently, in 2011, the International Association of Pancreatology gathered an international panel of experts to review existing criteria and develop consensus criteria and diagnostic algorithms for the diagnosis of AIP.13 The diagnostic algorithms incorporate a variety of evidence to make or exclude a diagnosis of AIP. Evidence can include a combination of the following: pancreatic imaging (both parenchymal and ductal findings), pancreatic histology, serology, extrapancreatic findings (histology or imaging), and response to steroid therapy. Using the evidence available from our case and the algorithms provided, an “indeterminate” diagnosis of AIP would have been made. This is based on the following evidence. CT findings of segmental/focal pancreatic enlargement with delayed enhancement, CBD strictures, and renal involvement. Based on the international consensus algorithms, a positive radiologic response to steroids would be required to diagnose AIP, with or without a positive renal biopsy. Therefore, a steroidal trial without renal biopsy, followed by repeat imaging to evaluate for response, would have been a reasonable therapeutic/diagnostic strategy. Given that the patient in our case had a positive clinical response to steroidal therapy and a biopsy-proven AIP-associated renal lesion, no follow up imaging was obtained. Autoimmune pancreatitis, and its extrapancreatic manifestations, respond very well to corticosteroid therapy, and standard treatment currently consists of 0.6 kg/mg/day of prednisolone for 2-4 weeks followed by a gradual taper to a low-maintenance dose that is continued for several years to prevent recurrence.14
CONCLUSION
Autoimmune pancreatitis is a chronic inflammatory disorder of unknown etiology associated with hypergammaglobulinemia that leads to fibrosis and organ dysfunction. The disease process can mimic pancreatic adenocarcinoma both clinically and radiographically, which can lead to difficulty in obtaining a diagnosis. Imaging plays a critical role in the diagnosis of AIP by characterizing both pancreatic and extrapancreatic involvement. Extrapancreatic involvement in AIP is not uncommon and most commonly affects the biliary system, kidneys, lymph nodes and gallbladder. Renal lesions associated with AIP appear to be relatively common, with recent publications reporting a prevalence of 14-39%.2,11-12 In cases where pancreatic findings are indeterminate or non-specific for AIP, the presence of extrapancreatic lesions should increase suspicion of AIP as a diagnosis. Awareness of AIP, its associated pancreatic and extrapancreatic findings, and the use of recently published diagnostic algorithms can help reach a diagnosis while potentially avoiding unnecessary procedures.
REFERENCES
- Okazaki K. Autoimmune pancreatitis: Etiology, pathogenesis, clinical findings and treatment. The Japanese experience. JOP.2005;6:89-96.
- Khalili K, Doyle DJ, Chawla TP et al. Renal cortical lesions in patients with autoimmune pancreatitis: a clue to differentiation from pancreatic malignancy. Eur J Radiol. 2008. 67(2):329-335.
- Vlachou PA, Khalili K, Jang HJ, et al. IgG4-related sclerosing disease: autoimmune pancreatitis and extrapancreatic manifestations. Radiographics. 2011;31(5):1379-1402.
- Takahasi N, Fletcher JG, Hough DM, et al. Autoimmune pancreatitis: Differentiation from pancreatic carcinoma and normal pancreas on the basis of enhancement characteristics at dual-phase CT. Am J Roentgenol. 2009;193(2):479-484.
- Irie H, Honda H, Baba S, et al. Autoimmune pancreatitis: CT and MR characteristics. Am J Roentgenol. 1998;170(5):1323-1327.
- Fujinaga Y, Kadoya M, Kawa S, et al. Characteristic findings in images of extra-pancreatic lesions associated with autoimmune pancreatitis. Eur J Radiol. 2010;76(2):228-238.
- Tan TJ, Ng YL, Tan D, et al. Extrapancreatic findings of IgG4-related disease. Clin Radiol.2014;69(2):209-218.
- Sarles H, Sarles JC, Camatte R, et al. Observation on 205 confirmed cases of acute pancreatitis, recurring pancreatitis, and chronic pancreatitis. Gut. 1965;6(6):545-559.
- Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38(10):982-984.
- Cornell LD, Chicano SL, Deshpande V, et al. Pseudotumors due to IgG4 immune-complex tubulointerstitial nephritis associated with autoimmune pancreatocentric disease. Am J Surg Pathol. 2007;31(10):1586-1597.
- Takahashi N, Kawashima A, Fletcher JG, et al. Renal involvement in patients with autoimmune pancreatitis: CT and MR imaging findings. Radiology. 2007;242(3):791-801.
- Triantopoulou C, Malachias G, Maniatis P, et al. Renal lesions associated with autoimmune pancreatitis: CT findings. Acta Radiol. 2010;51(6):702-707.
- Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40(3):352-358.
- Kamisawa T, Okazaki Y, Kawa S, et al. Japanese consensus guidelines for management of autoimmune pancreatitis: III. Treatment and prognosis of AIP. J Gastroenterol. 2010;45(5):471-477.
