Practicing safe use of nonionic, low-osmolarity iodinated contrast

Iodinated contrast agents have been used for many decades and play an important part in diagnostic and interventional procedures. Contrast media are generally safe, but allergy-like reactions to contrast can potentially become life threatening. To prevent adverse consequences, careful review of the patient’s history for medical necessity of contrast agent is needed. This article describes the epidemiology and types of contrast reactions, factors associated with aderse reactions, premedication to prevent adverse reactions, contrast-induced acute kidney injury, contrast use among pregnant and breast-feeding patients, and contrast extravasation management.
Iodinated contrast agents were developed in the 1920s.1,2 Starting as ionic, high osmolar contrast material (HOCM), contrast agents underwent further refinement during the 1960s with the introduction of nonionic compounds. Nonionic compounds do not dissociate in water; therefore, they are lower in osmolality. Low osmolar contrast media (LOCM) have undergone further evolution with increased hydroxyl groups replacing carboxyl groups for additional solubility in water. The lower osmolality and increased solubility in water lowers LOCM toxicity.2 Formerly used HOCMs have significantly higher incidence of adverse reactions compared to modern LOCMs.3,4,5 Usage of contrast materials has consequently increased exponentially as these agents have proven to be extremely safe with a low incidence of adverse reactions.1,6 Efforts aimed minimizing risks of contrast reactions are essential to providing high-quality healthcare.
Epidemiology
The incidence of allergy-like reactions to LOCMs is relatively rare, with estimates in the range of 0.2-3.1%.1,7 Most of these are classified as mild and moderate. There is increased incidence of adverse reactions among younger patients, which may be due to psychological effects.8
Risk factors exist that predispose patients to contrast reactions. Prior history of allergy to contrast increases the subsequent risk of reaction up to fivefold. Allergies to other medications, foods and environmental allergens also increase the risk; however, the magnitude of increased risk is unclear. Asthma and cardiovascular disease are also known risk factors for contrast reaction.5,6,9,10
Anxiety has been shown to increase the likelihood of contrast reaction. Advising patients of the side effects they may experience may help to minimize anxiety, and thereby reduce the risk of adverse reaction.8,11 Administering anxiolytics before contrast injection to reduce contrast reaction is not routinely practiced.
Controversy exists regarding the association of beta-blockers and adverse contrast reactions. Despite the lack of consensus on beta-blocker as a risk factor, studies indicate decreased efficacy of medications to treat contrast reactions if they occur.12,13
Nature of contrast reactions
Contrast reactions are not true allergic reactions. Symptoms such as urticaria and anaphylactoid reactions do not stem from true allergic reactions as one would expect after ingesting food or other immune-system-provoking substance. Examination of patients after contrast reaction has not revealed signs of antibody formation as one would expect after a true allergic reaction. Contrast agent molecules are theoretically too small to elicit antibody formation. Contrast agent reactions are therefore more properly classified as anaphylactoid reactions.5,13,14
Contrast reactions can be classified into 3 categories: mild, moderate and severe. Mild reactions include nausea, vomiting, limited urticaria and self-resolving vasovagal reaction. Moderate reactions include symptomatic urticaria, diffuse erythema and vasovagal reaction that responds to treatment. Severe reactions include diffuse edema, seizures and vasovagal severe bronchospasm.5,11
Table 1 outlines some considerations that should be taken in patients who report previous allergic reaction to IV contrast administration.
Premedication
Steroids and antihistamines are effective in minimizing adverse reactions in patients with risk factors. There are various protocols for premedication. One frequently used protocol is the Greenberger protocol, which includes prednisone 50mg at 13, 7, and 1 hr, and diphenhydramine 50mg at 1 hr prior to contrast injection.15,16,17 In settings where emergent contrast injection is required, higher doses of intravascular steroids can be used. As an example, methylprednisolone IV 40mg every 4 hrs and diphenhydramine IV 1 hr before contrast administration may be followed. As with any medication, steroids have side effects of their own; therefore, careful consideration of the risks versus benefits should be performed prior to administration.9,18,19
Seafood and drug allergies
Allergies to shellfish have long been mistakenly correlated with iodinated contrast agent allergy. There is no evidence regarding cross reactivity between shellfish allergy and anaphylactoid reaction to contrast. The previously held notion that patients with a history of seafood allergies are at increased risk for adverse reaction to contrast is no longer valid.9,14,20 A strong history of multiple drug allergies should increase awareness of the possible risk of a reaction, but there is no need to avoid injection of contrast media unless the allergy is to the IV contrast itself.5,21
Asthma and diabetes
A history of asthma has been thought to be a good predictor of increased risk; risk may be higher in patients with active asthma and/or bronchospasm. An inhaler should always be available when contrast media are being administered.21,22 Diabetic patients on metformin with normal renal function at baseline but with other comorbidities, such as liver or cardiac dysfunction, should stop metformin before receiving contrast and may resume 48 hrs afterwards if patient has no other intercurrent risk factors for renal damage. Patients with renal insufficiency should stop metformin and have repeat lab tests for return to baseline renal function or show signs of clinical stability prior to restarting metformin.23 Metformin does not need to be stopped prior to contrast administration in patients with normal renal function and without other comorbidities.11,24 Stopping metformin and/or rechecking creatinine levels 48 hours after the procedure may be unnecessary, because the risk of contrast-induced nephropathy in patients with normal renal function without other comorbidities is very low.
Observing patients for contrast reactions
Most adverse reactions occur within 5 minutes of contrast administration. Contrast is usually given through the intravascular route, which results in a rapid appearance of reactions. Contrast administered through the alimentary tract and intracavitary spaces can also cause adverse reactions, as some contrast is absorbed. Patients with moderate to severe contrast reactions should be observed until they demonstrate clinical stability.9,16
Creatinine check prior to IV contrast
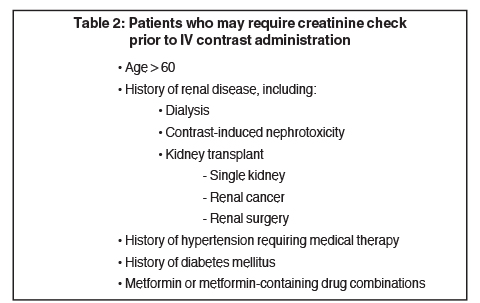
Not all patients require measurement of serum creatinine before receiving contrast, as individuals most at risk for contrast-induced acute kidney injury have a history of renal insufficiency.21 A recent study showed that patients with stable baseline serum creatinine of < 1.5 mg/dL are not at risk for renal injury following contrast administration.25 The general consensus is to allow contrast use in patients with creatinine ≤ 1.5 mg/dL, exercise caution in patients with creatinine in the range of 1.6 - 2.0 mg/dL, and to avoid contrast in patients with creatinine > 2.0 mg/dL. Table 2 lists risk factors for which the American College of Radiology recommends practicing caution in administering IV contrast.11
Creatinine cutoff for administering IV contrast
Elevated serum creatinine levels indicate poor renal function.26-28 Rising creatinine levels indicate that an insult to the kidney has occurred, with the elevation in creatinine lagging behind that of renal damage by 24-72 hrs. Among patients with compromised renal function and el evated creatinine, the correlation between acute renal damage and rise in creatinine becomes less clear. Recent studies suggest that acute renal injury as indicated by elevation in serum creatinine level seen after a contrast CT examination may not be due to the contrast material. Instead, the injury may be due to other confounding variables.29,30 Therefore, setting an absolute creatinine cutoff value for administering intravenous contrast is difficult. Creatinine cutoff of 2.0 mg/dL is probably safe for most patients with stable chronic renal insufficiency.11,31
Contrast-induced acute kidney injury
Contrast-induced acute kidney injury refers to the sudden decrease in renal function following contrast administration in the absence of other nephrotoxic agents. Although no formal criteria exist, a general guideline is elevation of serum creatinine by ≥0.5 mg/dL or ≥25% occurring within 48 hrs after contrast administration, and the absence of an alternative etiology.13,16,31,32 Serum creatinine is not a reliable marker for renal function, as values depend strongly on age, gender, muscle mass and nutritional state.33 A more accurate measurement is the glomerular filtration rate.11,34
A recent study showed that using an estimated glomerular filtration rate cutoff of 45 mL/min/1.73 m2 identified small but significantly more patients at risk of contrast induced acute kidney injury after contrast administration as well as patients at low risk who may be misclassified as being at risk for acute kidney injury when using the method of serum creatinine cutoff of 1.5 mg/dL.35
Prevention of contrast-induced acute kidney injury consists mainly of withholding contrast whenever possible and maximizing hydration with IV crystalline solution.10,23,36,37 Among patients undergoing cardiac catheterization, left ventricular end-diastolic pressure-guided fluid administration was useful in preventing renal injury.38 Controversy exists with regard to the usefulness of other medications to prevent acute kidney injury, such as sodium bicarbonate, N-acetylcysteine, fenoldopam, and theophylline.23 Studies show N-acetylcysteine decreases serum creatinine; however, there is no protection against renal failure.11,39,40
A recent study questions the widely held belief that contrast induces acute renal injury in patients with chronic renal insufficiency. The study concluded that no significant relationship exists between contrast administration and acute renal injury.29 Another study concluded no significant increased rates of acute renal injury after LOCM administration among critically ill patients.41 How much the impact of these results will change the practice of exercising caution in administering contrast to patients with decreased renal function remains to be seen.
Dialysis, pregnant and breast-feeding patients
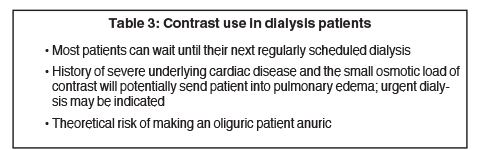
Patients with chronic kidney disease on dialysis may receive contrast. There is a theoretical risk of causing an oliguric patient anuric. For anuric patients, risks include causing fluid overload and pulmonary edema. Emergent dialysis is not needed unless patient shows signs of cardiopulmonary decompensation.11,42 Table 3 lists factors that need to be considered in a dialysis patient.
Iodinated contrast agents cross the placenta; therefore, the fetus will be exposed to small amounts of contrast. A limited number of studies show no evidence of mutagenic or teratogenic potential of contrast material. Association with hypothyroidism has not been shown in fetuses exposed to iodinated contrast.13,43, 44
Very small amounts of contrast are excreted into breast milk. The amount of contrast that a baby will ingest and absorb through the gastrointestinal tract is far less than that allowed for radiological examinations among infants. There is no need for mothers to discontinue breast-feeding after receiving contrast material. If the mother is very concerned about the unknown effects of contrast on her baby, she may express and discard her breast milk for 24 hrs after her examination. Virtually no contrast remains within the mother after 24 hrs.11, 45
Extravasation
Two main complications that can occur after contrast extravasation are skin necrosis and compartment syndrome. Extravasation into the soft tissues can cause necrosis of the surrounding skin as contrast elicits a foreign-body response and creates significant inflammation. Extravasation of relatively large amounts of contrast within a small volume of space such as the wrist or hand increases the risk for compartment syndrome.11,46-48
Conservative treatment includes application of cold or warm compresses, elevation of the affected extremity with serial pulse and sensation exams, and local massage.47 Cold compresses are thought to relieve pain and decrease inflammation. Warm compression is thought to increase blood flow to the area of extravasation and promote absorption of contrast. Patients should be monitored for several hours to make sure no further complications develop.11
Surgical consultation for management of contrast extravasation depends on patient’s symptoms. Previously, greater than 100 mL of extravasation was used as guideline for obtaining consultation. However, the most recent recommendation is to monitor for symptoms, such as increasing pain, skin necrosis and paresthesia in the affected limb, and decreased capillary refill.11,49,50
Conclusion
Careful consideration of contrast reactions and contrast-induced nephrotoxicity among select patients will minimize complications related to contrast use. Use of iodinated contrast agents as part of radiologic examination is safe among most patient populations, including pregnant patients and breast-feeding mothers, and should be utilized when medically necessary.
References
- Hunt CH, Hartman RP, Hesley GK. Frequency and severity of adverse effects of iodinated and gadolinium contrast materials: Retrospective review of 456,930 doses. AJR Am J Roentgenol. 2009;193:1124-1127.
- McClennan BL. Ionic and nonionic iodinated contrast media: Evolution and strategies for use. AJR Am J Roentgenol. 1990;155(2):225-233.
- Lasser EC, Berry CC, Talner LB, et al. Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material. N Engl J Med. 1987;317(14):845-849.
- Seong JM, Choi NK, Lee J, et al. Comparison of the safety of seven iodinated contrast media. J Korean Med Sci. 2013;28(12):1703-1710.
- Iyer RS, Schopp JG, Swanson JO, et al. Safety essentials: Acute reactions to iodinated contrast media. Can Assoc Radiol J. 2013;64(3):193-199.
- Pradubpongsa P, Dhana N, Jongjarearnprasert K, et al. Adverse reactions to iodinated contrast media: prevalence, risk factors and outcome-the results of a 3-year period. Asian Pac J Allergy Immunol. 2013;31(4):299-306.
- Kim MH, Lee SY, Lee SE, et al. Anaphylaxis to iodinated contrast media: Clinical characteristics related with development of anaphylactic shock. PLoS One. 2014;9(6):e100154.
- Gomi T, Nagamoto M, Hasegawa M, et al. Are there any differences in acute adverse reactions among five low-osmolar non-ionic iodinated contrast media? Eur Radiol. 2010;20(7):1631-1635.
- Schopp JG, Iyer RS, Wang CL, et al. Allergic reactions to iodinated contrast media: Premedication considerations for patients at risk. Emerg Radiol. 2013;20:299-306.
- Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: Updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21(12):2527-2541.
- American College of Radiology. American College of Radiology Manual on Contrast Media. 9th ed. Reston, Va; 2013.
- Greenberger PA, Meyers SN, Kramer BL, et al. Effects of beta-adrenergic and calcium antagonists on the development of anaphylactoid reactions from radiographic contrast media during cardiac angiography. J Allergy Clin Immunol. 1987;80(5):698-702.
- Namasivayam S, Kalra MK, Torres WE, et al. Adverse reactions to intravenous iodinated contrast media: A primer for radiologists. Emerg Radiol. 2006;12:210-215.
- Schabelman E, Witting M. The relationship of radiocontrast, iodine, and seafood allergies: A medical myth exposed. J Emerg Med. 2010; 39(5):701-707.
- Greenberger P, Patterson R, Kelly J, et al. Administration of radiographic contrast media in high-risk patients. Invest Radiol. 1980;15(6 Suppl):S40-S43.
- Shin MJ, Cho YJ. Management of adverse reaction to iodinated radiocontrast media. J Korean Med Assoc. 2012;55(8):779-790.
- Kim SH, Lee SH, Lee SM, et al. Outcomes of premedication for non-ionic radio-contrast media hypersensitivity reactions in Korea. Eur J Radiol. 2011;80(2):363-367.
- Kolbe AB, Hartman RP, Hoskin TL, et al. Premedication of patients for prior urticarial reaction to iodinated contrast medium. Abdom Imaging. 2014;39(2):432-437.
- Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep. 2005;5(1):28-31.
- Baig M, Farag A, Sajid J, et al. Shellfish allergy and relation to iodinated contrast media: United Kingdom survey. World J Cardiol. 2014;6(3):107-111.
- Bettmann MA. Frequently asked questions: Iodinated contrast agents. RadioGraphics. 2004;24:S3-S10.
- Morcos SK, Thomsen HS. Adverse reactions to iodinated contrast media. Eur Radiol. 2001;11:1267-1275.
- Thomsen HS, Morcos SK, Barrett BJ. Contrast-induced nephropathy: The wheel has turned 360 degrees. Acta Radiol. 2008;49(6):646-657.
- Andersen PE. Patient selection and preparation strategies for the use of contrast material in patients with chronic kidney disease. World J Radiol. 2012;4(6):253-257.
- Davenport MS, Khalatbari S, Dillman JR, et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267:94-105.
- Sandilands EA, Dhaun N, Dear JW, et al. Measurement of renal function in patients with chronic kidney disease. Br J Clin Pharmacol. 2013;76(4):504-515.
- Spanaus KS, Kollerits B, Ritz E, et al. Serum creatinine, cystatin C, and beta-trace protein in diagnostic staging and predicting progression of primary nondiabetic chronic kidney disease. Clin Chem. 2010;56(5):740-749.
- Dalton RN. Serum creatinine and glomerular filtration rate: perception and reality. Clin Chem. 2010;56(5):687-689.
- McDonald RJ, McDonald JS, Bida JP, et al. Intravenous contrast material-induced nephropathy: Causal or coincident phenomenon? Radiology. 2013;267:106-118.
- Moos SI, Stoker J, Nagan G, et al. Prediction of presence of kidney disease in a general patient population undergoing intravenous iodinated contrast enhanced computed tomography. Eur Radiol. 2014;24(6):1266-1275.
- Traub SJ, Kellum JA, Tang A, et al. Risk factors for radiocontrast nephropathy after emergency department contrast-enhanced computerized tomography. Acad Emerg Med. 2013;20(1):40-45.
- Keaney JJ, Hannon CM, Murray PT. Contrast-induced acute kidney injury: how much contrast is safe? Nephrol Dial Transplant. 2013;28(6):1376-1383.
- Davenport MS, Cohan RH, Khalatbari S, et al. The challenges in assessing contrast-induced nephropathy: where are we now? AJR Am J Roentgenol. 2014;202(4):784-789.
- Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009;20(11):2305-2313.
- Davenport MS, Khalatbari S, Cohan RH, et al. Contrast medium–induced nephrotoxicity risk assessment in adult inpatients: A comparison of serum creatinine level- and estimated glomerular filtration rate-based screening methods. Radiology. 2013;269:92-100.
- Geenen RW, Kingma HJ, van der Molen AJ. Contrast-induced nephropathy: Pharmacology, pathophysiology and prevention. Insights Imaging. 2013;4(6):811-820.
- Briguori C, Condorelli G. Hydration in contrast-induced acute kidney injury. Lancet. 2014;383(9931):1786-1788.
- Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: The POSEIDON randomised controlled trial. Lancet. 2014;383(9931):1814-1823.
- Hoffmann U, Fischereder M, Krüger B, et al. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15(2):407-410.
- Poletti PA, Saudan P, Platon A, et al. I.v. N-acetylcysteine and emergency CT: use of serum creatinine and cystatin C as markers of radiocontrast nephrotoxicity. AJR Am J Roentgenol. 2007;189(3):687-692.
- Ehrmann S, Badin J, Savath L, et al. Acute kidney injury in the critically ill: Is iodinated contrast medium really harmful? Crit Care Med. 2013;41(4):1017-1026.
- Younathan CM, Kaude JV, Cook MD, et al. Dialysis is not indicated immediately after administration of nonionic contrast agents in patients with end-stage renal disease treated by maintenance dialysis. AJR Am J Roentgenol. 1994;163(4):969-971.
- Rajaram S, Exley CE, Fairlie F, et al. Effect of antenatal iodinated contrast agent on neonatal thyroid function. Br J Radiol. 2012;85(1015):e238-242.
- Bourjeily G, Chalhoub M, Phornphutkul C, et al. Neonatal thyroid function: effect of a single exposure to iodinated contrast medium in utero. Radiology. 2010;256(3):744-750.
- Webb JA, Thomsen HS, Morcos SK, et al. The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol. 2005; 15(6):1234-1240.
- Belzunegui T, Louis CJ, Torrededia L, et al. Extravasation of radiographic contrast material and compartment syndrome in the hand: A case report. Scand J Trauma Resusc Emerg Med. 2011;19:9.
- Sbitany H, Koltz PF, Mays C, et al. CT contrast extravasation in the upper extremity: Strategies for management. Int J Surg. 2010;8(5):384-386.
- Selek H, Ozer H, Aygencel G, et al. Compartment syndrome in the hand due to extravasation of contrast material. Arch Orthop Trauma Surg. 2007;127(6):425-427.
- Tonolini M, Campari A, Bianco R. Extravasation of radiographic contrast media: Prevention, diagnosis, and treatment. Curr Probl Diagn Radiol. 2012;41(2):52-55.
- Wang CL, Cohan RH, Ellis JH, et al. Frequency, management, and outcome of extravasation of nonionic iodinated contrast medium in 69,657 intravenous injections. Radiology. 2007;243(1):80-87.
