Part 1: Classic signs in gastrointestinal radiology














Over the years, radiologists have established many classic imaging signs that represent visual manifestations of myriad underlying pathophysiologic processes. Though many of these signs were initially described on plain films, they are still used daily by radiologists reviewing images from cross-sectional modalities. For example, classic plain-film signs, such as the “accordion sign,” are also commonly cited today when seen on multidetector CT (MDCT) images.
The names of many classic radiologic signs derive from analogues to objects commonly encountered in everyday life; eg, the “comb” sign. The use of familiar objects to describe visual findings enables radiologists both to arrive at a correct diagnosis and to effectively convey such diagnostic findings to clinicians.
The goal of this article is to review an array of classic signs associated with gastrointestinal tract pathologies whose imaging manifestations resemble everyday objects. The “football” and “cobblestone” signs, for instance, are especially well known to the authors in Boston.Signs that do not conjure a resemblance to familiar objects, such as the “moulage” and the “colon cut-off” signs, are not covered. This article organizes the gastrointestinal signs from proximal to distal within the gastrointestinal tract. The hepatobiliary and pneumoperitoneum signs are then reviewed.
Bird’s beak sign
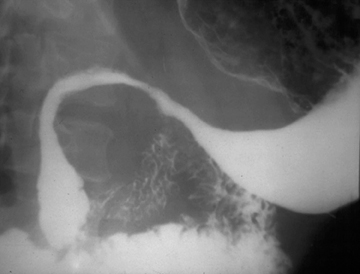
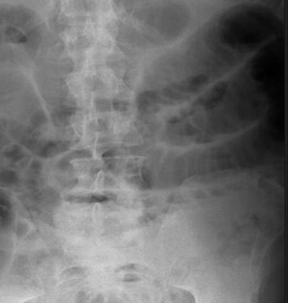
The “bird’s beak” sign is a classic finding on esophagrams; it describes a dilated proximal esophagus with a smooth-tapered, distal esophagus at the level of the esophageal hiatus in the setting of achalasia.1 The smooth tapering of the distal esophagus resembles the beak of a bird (Figure 1). On imaging and manometry, achalasia is further characterized by esophageal aperistalsis and failure of the lower esophageal sphincter to relax.
There are both primary and secondary forms of achalasia. Primary achalasia, the more common etiology, is idiopathic. The lack of lower esophageal sphincter relaxation is likely due to a loss of inhibitory neurons in the esophageal myenteric plexus. Proposed causes include neuronal degeneration, viral infection, genetic inheritance, and autoimmune disease.2 Secondary achalasia affects patients much less commonly and can be caused by entities such as esophageal carcinoma and Chagas disease.
Corkscrew sign
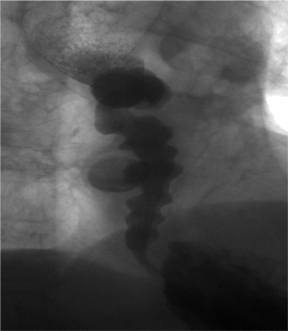
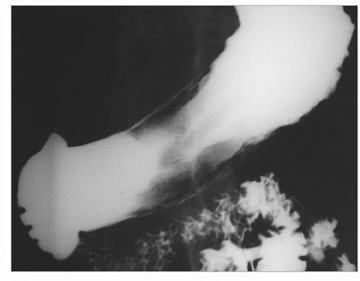
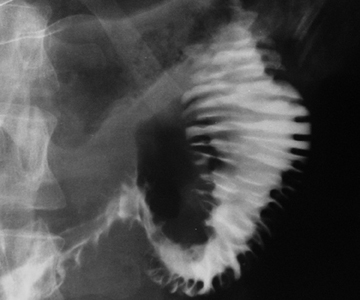
The “corkscrew” sign is the visual manifestation of lumen-obliterating, simultaneous, nonperistaltic contractions within the esophagus.
These abnormal contractions of varying amplitude occur in diffuse esophageal spasm, a rare esophageal motility disorder.3 Diffuse esophageal spasm is characterized on manometry by periods of normal peristalsis followed by simultaneous, repetitive, ineffective contractions. These abnormal contractions segment the normal esophageal lumen, mimicking a corkscrew on barium studies of the esophagus (Figure 2).4
Double-barrel esophagus
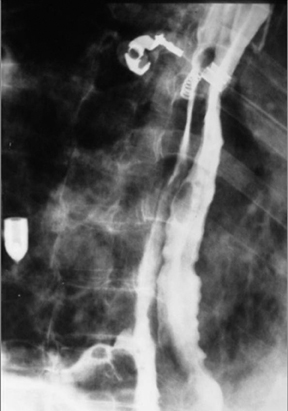
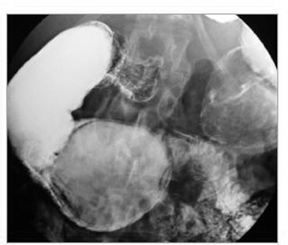
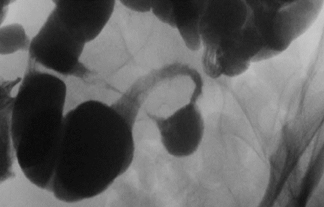
The term “double-barrel esophagus” classically refers to the radiographic appearance of a dissection between the esophageal mucosa and submucosa without perforation. This rare condition was first described by Marks and Keet in 1968.5 The double-barrel radiographic appearance (Figure 3) of the esophagus is due to the visualization of a barium-filled, intramural dissecting channel separated from the true esophageal lumen by a lucent line, the mucosal stripe.6
Intramural esophageal dissection is most commonly seen in middle-aged or elderly women. This entity can occur in the setting of a coagulopathy, emetogenic injury, trauma, instrumentation, ingestion of foreign bodies and, rarely, spontaneously.7 The double-barrel esophagus appearance can also be seen with intramural esophageal abscess, intraluminal diverticulum, or esophageal duplication.6
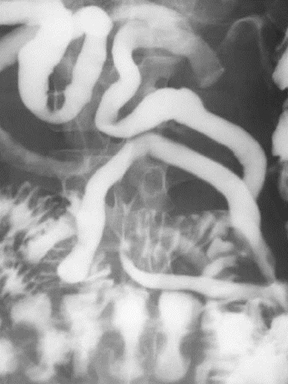
Bull’s eye lesions
Lesions within the stomach forming central collections of oral contrast within ulcerated intramural masses can produce a target or bull’s eye appearance (Figure 4) on upper gastrointestinal barium examinations. This bull’s eye appearance has been described with many disease processes.This differential diagnosis is broad and includes gastric metastatic lesions from melanoma and lymphoma.8 Kaposi’s sarcoma and carcinoid tumors have also been documented to produce a gastric bull’s eye appearance.8,9 Rarely, gastric lipomas may also ulcerate and produce a bull’s eye appearance.10
Ram’s horn
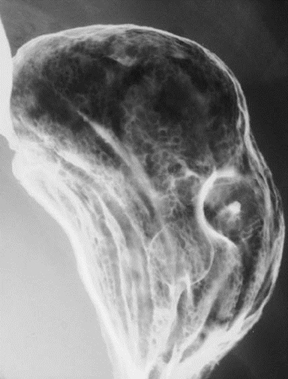
Crohn’s disease affects the stomach and duodenum in 0.5% to 4.0% of patients.11 The antrum is the gastric region most frequently involved. Crohn’s disease of the stomach leads to gastric deformity, causing a tubular shape, conical narrowing, and limited distensibility of the stomach.12 These changes may give the stomach an unusual shape resembling the horn of a ram (Figure 5). The presence of this gastric appearance in a young patient without an established diagnosis of Crohn’s disease should lead to a thorough workup to confirm or exclude the diagnosis.11
Leather bottle stomach
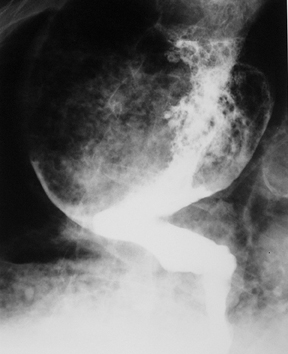
Primary scirrhous adenocarcinoma of the stomach spreads predominantly in the submucosa and muscularis propria. This type of tumor spread leads to a marked desmoplastic reaction and a thickened, stiff gastric wall and a narrow lumen.13 Scirrhous tumors constitute 5% to 15% of all gastric carcinomas.14 The stiff, nondistensible wall gives the stomach a leather bottle appearance,also known as linitis plastica (Figure 6).
Scirrhous adenocarcinoma is thought to arise near the pylorus and spread proximally.15 These tumors can eventually demonstrate diffuse involvement of the entire stomach. Other patients occasionally demonstrate localized tumors confined to the distal antrum.16
The differential diagnoses for the appearance of a leather bottle stomach include scirrhous metastases from lung, breast, colon, and pancreatic carcinomas; lymphoma; or inflammatory conditions, such as Crohn’s disease, sarcoidosis, and syphilis.
Windsock sign
Intraluminal duodenal diverticulum is a rare congenital cause of duodenal obstruction. Nelson first described the entity in 1947.17 These intraluminal diverticula are believed to arise from an improper luminal recanalization of the foregut in the 7th week of embryogenesis. A residual tissue diaphragm may span the entire circumference of the duodenum and only allow passage of enteric contents through fenestrations.18
The presence of these rare diverticula can be seen on upper gastrointestinal series and MDCT scans demonstrating the pathognomonic“windsock” sign. This windsock appearance is most commonly located in the second portion of the duodenum and consists of the barium-filled diverticulum that lies entirely within the duodenum (Figure 7). The windsock appearance is formed by passive elongation of the intraluminal diverticulum due to continual peristalsis of the duodenum.18,19
Double bubble sign
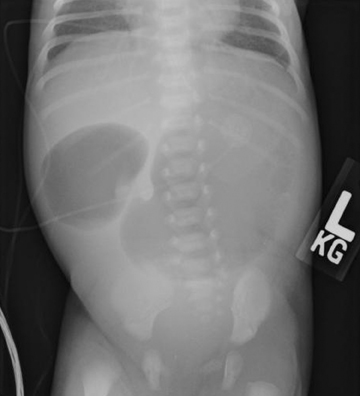
The “double bubble” sign represents the appearance of 2 gas-filled structures in the upper abdomen of newborns and infants on plain filmsof the abdomen (Figure 8). The left-sided, proximal bubble is the distended gas and fluid-filled stomach. The second, right-sided, more distal bubble is the distended duodenum.20,21
The double bubble sign indicates the presence of duodenal obstruction that can be caused by a number of intrinsic or extrinsic etiologies.The intrinsic causes include duodenal webs, duodenal atresia, and duodenal stenosis. The extrinsic etiologies include a preduodenal portal vein, malrotation of the gut with a midgut volvulus or by Ladd bands, or an annular pancreas.22
Duodenal atresia is the causative entity most commonly linked with a double bubble sign. Duodenal atresia is found in 1 in 10,000 newborns and is typically associated with other congenital anomalies; 30% of children with duodenal atresia have Down’s syndrome.23
Whirlpool sign
The “whirlpool” sign is found on both cross-sectional imaging as well as abdominal ultrasound in the presence of midgut volvulus. The whirlpool appearance represents the swirling pattern of the gut and the superior mesenteric vein as they wrap around the superior mesenteric artery (SMA) in a clockwise rotation (Figure 9).24
Normally, the midgut undergoes a 270-degree, counterclockwise rotation during embryologic development. Malrotation of the midgut represents a spectrum of developmental anomalies that result in either an insufficient or total lack of counterclockwise rotation of the midgut around the axis of the SMA. These anomalies all lead to a shortened mesenteric base.25 The shortened mesentery predisposes to volvulus that may result in bowel obstruction. Midgut volvulus is the most common complication of malrotation of the small bowel in adults. It is the clockwise rotation of the bowel loops that result in the whirlpool sign on cross-sectional imaging.24
String of pearls
The “string of pearls” sign indicates the presence of a small-bowel obstruction. This sign is also commonly referred to as the “string of beads” sign. It represents a row of small gas bubbles oriented in a relatively linear fashion within the abdomen on plain films (Figure 10).26
The observed rows of gas bubbles represent gas trapped between the valvulae conniventes of the nondependent wall of small bowel. These loops of small bowel are dilated and filled with fluid in the setting of a small-bowel obstruction, thus the meniscal effect of the surrounding fluid gives these pockets of gas a rounded or ovoid appearance. The string of pearls sign can be seen in both upright and decubitus plain radiographs in the setting of a small-bowel obstruction.27
Stack of coins
The “stack of coins” sign typically indicates the presence of a small-bowel hematoma. This sign is seen on plain films or MDCT images and represents adjacent, thickened folds with sharp demarcation and crowding of the valvulae conniventes (Figure 11).28
Small-bowel hematomas are being seen with increasing frequency due to the prevalence of anticoagulation therapy. Over-anticoagulation with warfarin is the most common cause of spontaneous intramural small-bowel hematoma. Other causes that may lead to the stack of coins sign include idiopathic thrombocytopenic purpura, leukemia, pancreatitis, pancreatic cancer, hemophilia, lymphoma, myeloma, chemotherapy, and vasculidites.29
String sign
In the setting of Crohn’s disease, the terminal ileum often becomes markedly stenotic secondary to bowel-wall inflammation and fibrosis.This results in the lumen of this portion of the small bowel resembling a piece of string on plain radiographs after ingestion of high-density oral contrast material (Figure 12).30
The string sign represents the marked narrowing of the terminal ileum lumen secondary to symmetric, transmural granulomatous inflammation and subsequent fibrotic thickening of the bowel wall.31 Bowel-wall thickening is the most common manifestation of Crohn’s disease on MDCT scans, occurring in up to 82% of patients.32
Ribbon sign
Donor lymphoid cells damage host tissues in graft-versus-host disease (GVHD). The organs most commonly affected by GVHD include the gastrointestinal tract, liver, and skin. Marked bowel-wall thickening can occur both in the small and large bowel.33
Fluoroscopic examinations performed with high-density oral contrast material in patients with GVHD of the GI tract may demonstrate marked fold thickening, luminal narrowing, separation of folds, and ultimately complete effacement of the valvulae conniventes. The latter causes the so-called “ribbon sign” (Figure 13).34,35 Colonic findings in GVHD include luminal narrowing, wall thickening, and loss of haustration. Evaluation with MDCT may demonstrate diffuse wall thickening and luminal narrowing of both the small and large bowel.33
The ribbon bowel appearance can also occur with multiple other clinical settings, such as infection, irradiation, allergy, ischemia, ingestion of corrosives or medications, amyloid, mastocytosis, lymphoma, Crohn disease, and celiac disease.36
REFERENCES
- Sabharwal T, Cowling M, Dussek J, et al. Balloon dilatation for achalasia of the cardia: Experience in 76 patients. Radiology. 2002;224:719-724.
- Park W, Vaezi MF. Etiology and pathogenesis of achalasia: The current understanding. Am J Gastroenterol. 2005;100:1404-1414.
- Richter JE, Castell DO. Diffuse esophageal spasm: A reappraisal. Ann Intern Med. 1984;100:242-245.
- Chen YM, Ott DJ, Hewson EG, et al. Diffuse esophageal spasm: Radiographic and manometric correlation. Radiology.1989;170:807-810.
- Marks IN, Keet AD. Intramural rupture of the oesophagus. Br Med J. 1968;3:536-537.
- Eisenberg RL. Gastrointestinal radiology: A pattern approach. 4th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2002:138-145.
- Chiu HH, Lee SY. Intramural dissection of the esophagus: Endoscopic findings. J Intern Med Taiwan. 2006;17:302-305.
- Dunnick NR, Harell GS, Parker BR. Multiple “bull’s-eye” lesions in gastric lymphoma. Am J Gastroenterol. 1976;126:965-969.
- Richey LE, Cooley RN. Kaposi’s sarcoma: The radiographic manifestations of involvement of the stomach. Gastroenterology.1963;44:195-198.
- Park SH, Han JK, Kim TK, et al. Unusual gastric tumors: Radiologic-pathologic correlation. Radiographics. 1999;19:1435-1446.
- Fielding JF, Toye DM, Beton DC, Cooke WT. Crohn’s disease of the stomach and duodenum. Gut. 1970;11:1001-1006.
- Farman J, Faegenburg D, Dallemand S, Chen CK. Crohn’s disease of the stomach: The “ram’s horn” sign. Am J Gastroenterol.1975;1:242-251.
- Levine MS, Kong V, Rubesin SE, et al. Scirrhous carcinoma of the stomach: Radiologic and endoscopic diagnosis. Radiology. 1990;175: 151-154.
- Moore JR. Gastric carcinoma: 30 year review. Can J Surg. 1986;29:25-28.
- Raskin MM. Some specific radiological findings and consideration of linitis plastica of the gastrointestinal tract. CRC Crit Rev Clin Radiol Nucl Med. 1976;8:87-105.
- Balthazar EJ, Rosenberg H, Davidian MM. Scirrhous carcinoma of the pyloric channel and distal antrum. AJR Am J Roentgenol.1980;134:669-673.
- Nelson WI. Congenital diaphragm of the duodenum: Case report with preoperative x-ray studies. Minn Med. 1947;30:745-752.
- Johnston P, Desser TS, Bastidas JA, Harvin H. MDCT of intraluminal “windsock” duodenal diverticulum with surgical correlation and multiplanar reconstruction. Am J Gastroenterol. 2004;183:249-250.
- Govaere F, Mortele KJ, Hesse U, et al. Giant intraluminal duodenal diverticulum: Conventional barium study and computed tomography findings. JBR-BTR. 2000;83:71-72.
- Traubici J. The double bubble sign. Radiology. 2001;220:463-464.
- De Backer AI, Mortele KJ, Ponomarenko N, De Keulenaer B. Images in clinical radiology. Double bubble sign in the newborn. JBR-BTR. 2003;86:306.
- Leonidas JC, Berdon W. The neonate and young infant: The gastrointestinal tract. In: Silverman FN, Kuhn JP, eds. Caffey’s Pediatric X-Ray Diagnosis. 9th ed. St Louis, Mo: Mosby; 1993:2048-2055.
- In: Rudolph AM, Hoffman JIE, Rudolph C. Rudolph’s Pediatrics. 20th ed. Stamford, CT: Appleton and Lange; 1996:1069.
- Epelman M. The whirlpool sign. Radiology. 2006;240:910-911.
- Bernstein SM, Russ PD. Midgut volvulus: A rare cause of acute abdomen in an adult patient. Am J Gastroenterol. 1998;171:639-641.
- Maglinte DD, Reyes BL, Harmon BH, et al. Reliability and role of plain film radiography and CT in the diagnosis of small-bowel obstruction. Am J Gastroenterol. 1996;167:14511455.
- Nevitt PC. The string of pearls sign. Radiology. 2000;214:157-158.
- Lane MJ, Katz DS, Mindelzun RE, Jeffrey RB Jr. Spontaneous intramural small bowel haemorrhage: Importance of non-contrast CT. Clin Radiol. 1997;52:378-380.
- Abbas MA, Collins JM, Olden KW. Spontaneous intramural small-bowel hematoma: Imaging findings and outcome. Am J Gastroenterol.2002;179:1389-1394.
- Nelson SW. Some interesting and unusual manifestations of Crohn’s disease (“regional enteritis”) of the stomach, duodenum, and small intestine. Am J Roentgenol Radium Ther Nucl Med. 1969;107:86-101.
- Cotran RS, Kumar V, Robbins SL. Diseases of Immunity. In Schoen, FJ, ed. Robbins Pathologic Basis of Disease. 5th ed. Philadelphia, Pa:W.B. Saunders; 1994:801-804.
- Goldberg HI, Gore RM, Margulis AR, et al. Computed tomography in the evaluation of Crohn disease. Am J Gastroenterol. 1983;140:277-282.
- Jones B, Kramer SS, Sara R, et al. Gastrointestinal inflammation after bone marrow transplantation: Graft-versus-host disease or opportunistic infection? Am J Gastroenterol. 1988;150:277-281.
- Jones B, Wall S. Gastrointestinal disease in the immunocompromised host. Radiol Clin North Am. 1992;30:555-577.
- Kalantari BN, Mortele KJ, Cantisani V, et al. CT features with pathologic correlation of acute gastrointestinal graft-versus-host disease after bone marrow transplantation in adults. Am J Gastroenterol. 2003;181:1621-1625.
- Gramm HF, Vincent ME, Braver JM. Differential diagnosis of tubular small bowel. Curr Imaging.1990;2:62.
