Normal Coronary Anatomy and Anatomic Variations
Images








Dr. Fiss graduated summa cum laude from La Salle University in Philadelphia, PA in 1996. He graduated from The Medical College of Pennsylvania/Hahnemann School of Medicine, Alpha Omega Alpha, in 2000. He received the William Likoff Award for Clinical Excellence. He completed his Internal Medicine Residency training at Temple University Hospital, Philadelphia in 2003. He spent the following year as a Fellow in Cardiovascular Research at Temple University School of Medicine after submitting a grant that was successfully funded by the Heart Failure Society of America. He is currently a Fellow in Cardiovascular Disease at Temple University Hospital. After completing his fellowship, Dr. Fiss plans to continue his academic endeavors with further training in interventional cardiology.
With the evolution of multislice, multidetector cardiac CT, noninvasive imaging of small moving structures, such as coronary arteries, has become possible. Until now, imaging of the coronary arterial tree has been limited to cine arteriograms. With cardiac CT, which provides detailed anatomic information, a firm understanding of gross anatomy with an appreciation of normal origin, branching, myocardial distribution, and adjacent structures is essential for accurate image interpretation. Furthermore, without a detailed appreciation of normal anatomy, coronary artery anomalies may go undiagnosed. This article will review normal coronary arterial anatomy from both anatomic and clinical standpoints, anatomic anomalies of the coronary arteries, and applications of cardiac CT in patient evaluation.
The anatomy of the coronary vessels has been described in detail for at least 3 centuries. Strict anatomic descriptions of the coronary vessels are available from standard text- books of gross anatomy. However, most of the textbook descriptions are based on views and perspectives obtained from tissue, emphasizing landmarks that are visible on the gross specimen. These landmarks are not easily recognizable on traditional cine radiographic images.
Until recently, most radiographic and clinical descriptions of the coronary arteries have been based on cine coronary arteriograms. When studying a cine arteriogram, however, a physician uses an entirely different set of reference points and is less inclined to emphasize landmarks that are seen only on the gross specimen. Because these anatomic landmarks are not easily recognized with traditional arteriography, clinicians have adopted slightly different nomenclature to describe normal coronary anatomy, concentrating more on vessels with clinical rather than anatomic significance.
Cardiac computed tomography (CT) provides detailed anatomic information, but its use requires a firm understanding of gross coronary anatomy. Furthermore, detailed appreciation of the "normal" origin, course, branching, adjacent structures, and myocardial distribution of these vessels is vital so that variations of the "normal" anatomy can be more easily recognized and applied to clinical practice.
This article will review normal coronary arterial anatomy from both anatomic and clinical standpoints, anatomic anomalies of the coronary arteries, and applications of cardiac CT in patient evaluation.
Normal coronary artery anatomy
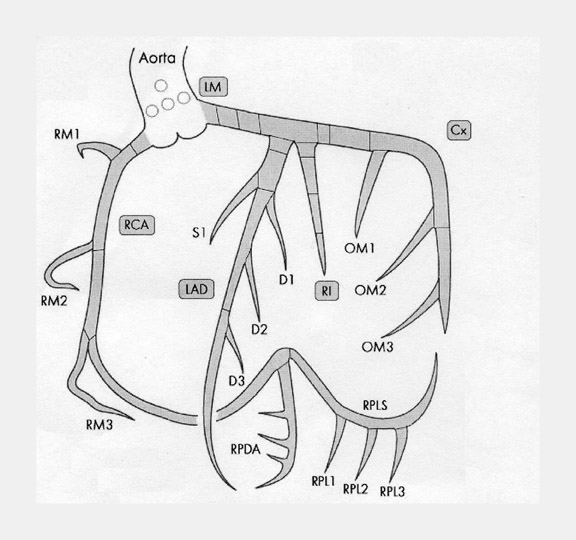
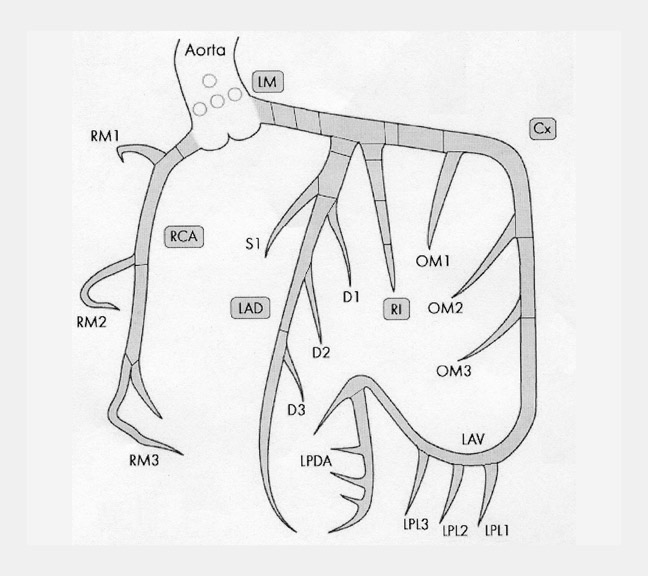
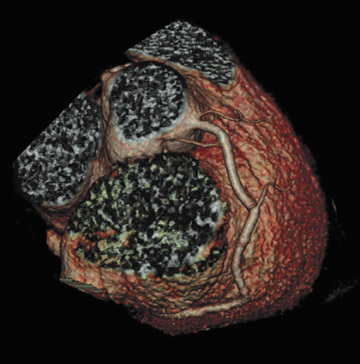
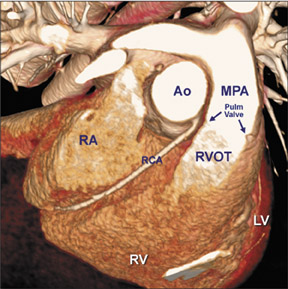
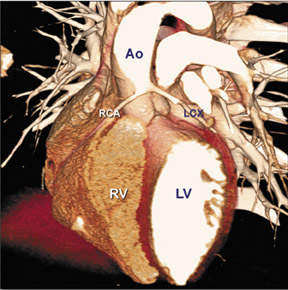
There are many variations of "normal" that are not considered "anomalous." Additionally, an understanding of which arterial branch perfuses which myocardial segment is germane to the physician when making individual patient care decisions. As a result, the concept of dominance has been adopted into the clinical vernacular to describe which artery gives rise to the posterior descending artery (PDA), the posterolateral artery (PLA), and the atrioventricular (AV) nodal artery, which, in turn, supply the inferior aspect of the interventricular septum, the inferior aspect of the left ventricle, and the AV node, respectively. If these arteries originate from the right coronary artery (RCA), the circulation can be classified as "right-dominant" (Figure 1). 1 Alternatively, if supplied by the left circumflex artery (LCx), the circulation can be classified as "left dominant" (Figure 2). In such patients, the right coronary artery is quite small and supplies only the right atrium and right ventricle. In the case of "co-dominant" circulation, the right coronary artery supplies the PDA and terminates. The left circumflex artery supplies the PLA with an occasional parallel posterior descending branch that supplies the inferior interventricular septum. Approximately 85% of the general population is right-dominant, 8% are left-dominant, and 7% are co-dominant. 2
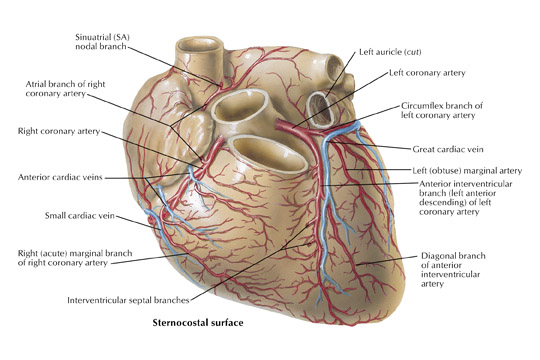
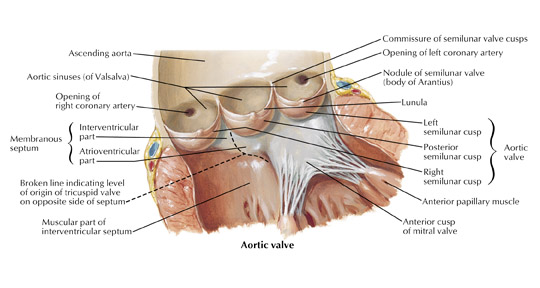
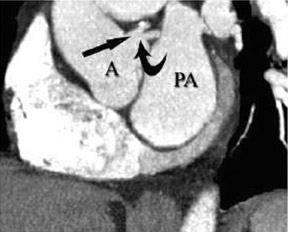
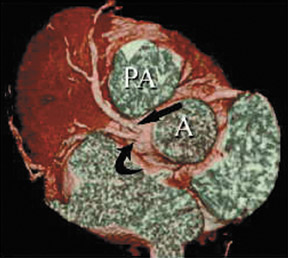
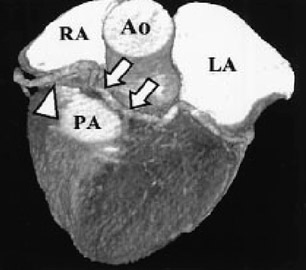
In the vast majority of people, there are two main coronary arteries, right and left, which arise from separate ostia in the aorta. The bulbar aortic sinus and the proximal ascending aorta comprise the aortic root. A slight circumferential thickening, known as the sinotubular ridge (sinotubular junction), marks the separation of these two structures (Figure 3). The bulbous sinus and the 3 aortic cusps merge to form the sinuses of Valsalva. The right sinus of Valsalva lies right and anterior in the aortic root and contains the right aortic semilunar cusp, whereas the left sinus of Valsalva lies left and posterior in the aortic root and contains the left aortic semilunar cusp. The posterior sinus of Valsalva lies posterior to the right sinus and contains the noncoronary aortic semilunar cusp. The coronary ostia are usually located below the sinotubular ridge, within the sinus of Valsalva, centrally located between the commissural attachments of the aortic cusps (Figure 4). 3 The ostium of each coronary artery tends to form a slight funnel, with the diameter of the left main coronary artery at its ostium slightly larger than that of the right coronary artery (mean 4.0 versus 3.2 mm). 4
Right coronary artery
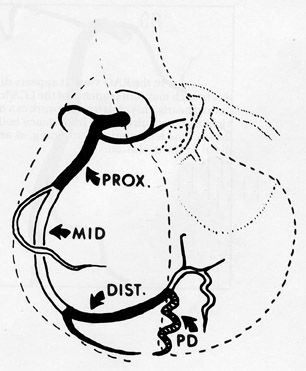
The right coronary artery, as it emerges from its ostium, lies deep in the epicardial fat between the pulmonary conus and the right atrium and is somewhat obscured by the right atrial appendage. Covered by fat, its course runs deep in the right atrioventricular sulcus with the right atrium (RA) cephalad and the right ventricle (RV) caudad (Figure 1B). It continues to course downward around the acute margin of the heart and then posteriorly, remaining in the AV sulcus until it reaches the interventricular sulcus (which separates the right and left ventricles) at the crux (the point where the interatrial sulcus crosses the interventricular sulcus) (Figure 1C). During its course, it gives off a variable number of branches. The RCA branches tend to take off at right angles while those of the left anterior descending artery (LAD) tend to separate from their parent artery at more acute angles (Figure 1B). For simplification, the branches of the RCA will be described in the usual order of branching from proximal to distal. The conus artery, right atrial branches, right ventricular branches, interventricular septal branches, AV nodal branches, and left ventricular branches will be discussed. Clinicians, to more accurately describe lesion location, divide the RCA into proximal (ostium to first main RV branch), mid (first main RV branch to the acute margin), and distal (acute margin to the crux) (Figures 1A and 5).
The conus artery (infundibular artery, adipose artery, third coronary artery, arteria of Vieussens) when present, takes a semicircular course away from the RCA on the epicardial anterior surface of the right ventricle at the level of the pulmonary valve. It terminates in small "twigs" near the superior aspect of the anterior interventricular sulcus. A separate ostium gives rise to the conus artery in 23% to 51% of patients and will be discussed further in later sections. 2 If not, the conus is the most proximal branch of the RCA (Figure 6). Interest in the conus branch stems from the fact that it often has a separate ostium and also from the observation that it often forms a vascular anastomotic bridge with a corresponding branch from either the left main or proximal LAD forming the circle of Vieussens. This bridge may play a role as a collateral pathway to the LAD.
The atrial branches of the RCA are very variable in number, size, and location. The right atrial branches are named according to their origin from the RCA as it travels downward in the atrioventricular sulcus (anterior, marginal/intermediate, or posterior) and are usually small in caliber (≤1 mm). 5 The main atrial artery is usually the atrial branch that terminates in the sinoatrial node. The artery to the sinoatrial node arises from the RCA in 60% of subjects (40% from the left circumflex artery). It usually arises from the proximal (anterior) segment of the RCA and ascends posteriorly along the body of the right atrium behind the aorta to reach the anterior aspect of the interatrial groove (Figure 6). During its course, it gives off branches to both atria and penetrates into the interatrial septum. The artery terminates by partially or completely encircling the lower portion of the superior vena cava, giving off branches called the ramus cristae terminalis, which terminate in the sinoatrial node. Other small atrial branches may arise from the RCA but are often difficult to visualize angiographically because of their small caliber and have limited clinical significance.
The right ventricular branches of the RCA take a looping course as they emerge from the atrioventricular sulcus at nearly right angles from the RCA and course over the anterior, marginal, and posterior surface of the right ventricle. The nomenclature used to describe these vessels depends on their origin and course. Usually, they are simply referred to by clinicians as right ventricular branches. The conus artery usually supplies the upper anterior portion of the right ventricle. In approximately 65% to 85% of subjects, 1 or 2 right ventricular branches arise from the anterior segment of the RCA. 6 In 4% to 9% of cases, no definite anterior right ventricular branch is present. In this case, the anterior portion of the right ventricle is supplied by the conus artery and a right ventricular marginal branch. The anterior and marginal right ventricular branches usually run a parallel course over the right ventricular surface (Figure 1B). The posterior right ventricular branches are usually small and vary in number.
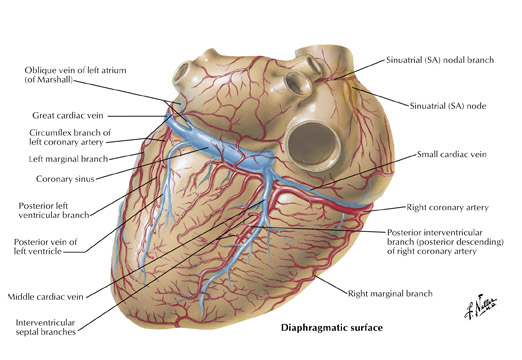
The inferior aspect of the interventricular septum is supplied by the posterior descending artery. As described above, the posterior descending artery usually arises as a branch or continuation of the RCA where the atrioventricular sulcus meets the interventricular sulcus (the crux) on the posterior surface of the heart. The posterior descending artery courses along the posterior aspect of the interventricular sulcus a variable distance toward the apex (Figure 1C). The distance the posterior descending artery descends towards the apex is inversely related to the posterior extension of the LAD, which often wraps around the apex and courses cephalad in the posterior interventricular sulcus. Arising from the posterior descending artery, a variable number of branches penetrate upward into the inferior portion of the interventricular septum. They are usually oriented to the right side of the inferior septum and are short in length (<15 mm) when compared with downward coursing septal branches from the LAD (40 to 80 mm). The posterior descending artery may give off parallel branches to the right or left. Also, occasionally branches of an acute marginal branch may supply portions of the inferior aspect of the interventricular septum. Of note, the ramus cristae supraventricularis or superior septal artery has been described in 12% to 20% of human hearts and is more common in males. This normal variant arises from the proximal right coronary artery and descends through the superior septal border. Once within the interventricular septum, it courses down the middle of the septum to the level of the atrioventricular node or may continue down the septum, replacing or supplementing the septal branches arising from both the left anterior descending and posterior descending arteries.
The artery to the AV node usually arises from the RCA and less frequently from the left circumflex artery, depending on which artery crosses the crux. The atrioventricular node artery arises from the RCA in 85% and 91% of male and female subjects, respectively, and from the left circumflex artery in 13% and 4.5% of males and females, respectively. The AV node artery arises from both arteries in 2% of subjects. 7,8 The RCA (or left circumflex artery) as it courses in the AV sulcus makes a U-shaped bend at the crux around the posterior interventricular vein by penetrating within the myocardium and emerging again on the other side of the vein. The AV nodal artery arises from the apex of the U-turn and takes a straight course 2 or 3 cm cephalad, terminating in right angle branches to the AV node.
The left ventricular branches of the RCA are known as the posterolateral branches. These branches extend to the adjacent portion of the left ventricle and arise at right angles from the posterior descending artery. Angiographically, they represent a continuation of the RCA beyond the posterior descending artery. These branches traverse the interventricular septum and supply the inferior wall of the left ventricle.
Left coronary artery
Left main artery
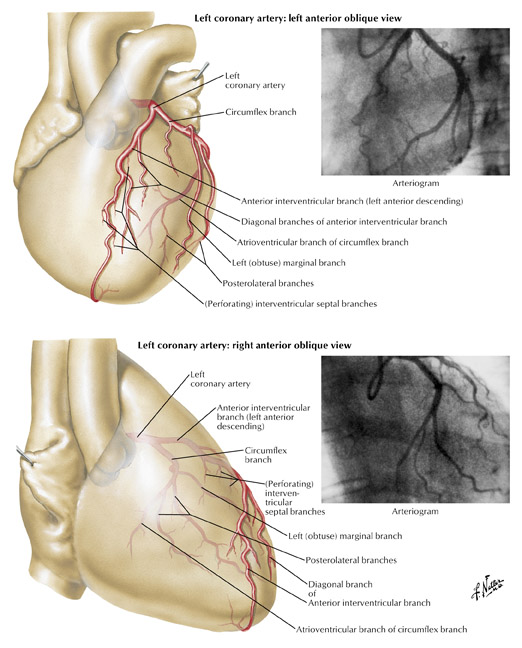
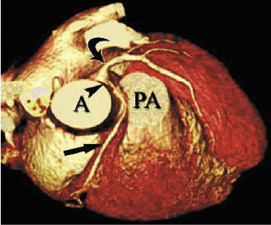
The left coronary (left main) artery arises from the left sinus of Valsalva and courses laterally between the base of the pulmonary trunk and left atrium (Figure 7). The length of the left main, in general, varies from 2 to 12 mm but may be up to 30 mm. Its diameter, ranging from 5 to 10 mm, is generally inversely related to its length. Cardiac multislice CT (MSCT) provides excellent visualization of the left main coronary artery. In a study assessing quantitative parameters of image quality with 64-slice CT, Ferencik et al 9 described the length of the left main as 12 ± 6 mm and visualized the entire left main with no motion artifact in 100% of patients studied (Figure 8A).The left main usually has 2 major branches, the LAD and left circumflex arteries. Occasionally, the left main trifurcates into the LAD, left circumflex, and a third (intermediate) artery. This third branch originates between the angle formed by the LAD and the left circumflex arteries and has various names, including "ramus intermediate," "median artery," "left diagonal artery," and "straight left ventricular artery."
Left anterior descending artery
The LAD is, for practical purposes, a continuation of the left main artery. It passes to the left of the pulmonary trunk, travels into the upper portion of the interventricular sulcus, and continues toward the apex of the heart. As it turns around the pulmonary artery and begins its downward course into the interventricular sulcus, the LAD forms a 90˚angle, often highlighted by the origin of the second diagonal branch (Figure 7). Surgically this location is very important, as it represents the point at which the LAD becomes amenable to bypass. How far the left anterior artery extends is variable, but it usually at least continues to the apex. Occasionally, the LAD artery bifurcates into 2 parallel vessels, which descend along the edges of the interventricular sulcus toward the apex. During its course, the LAD is often covered by superficial muscle fibers, which run at right angles to the vessel, creating what is known as a "myocardial bridge."
Clinicians divide the LAD into 3 segments: proximal (from origin to first ma-jor septal perforating branch), mid (from the origin of the first septal perforator to the 90˚ angle described above at the second diagonal branch), and distal (from diagonal 2 to vessel end) (Figure 1A).
The left ventricular branches of the LAD are known as the diagonal branches, because they branch from their parent vessel at acute angles and extend over the left ventricle in a diagonal fashion toward the acute margin and the apex. They run parallel to one another and are variable in number (2 to 9). If a ramus intermediate artery is present, the diagonal vessels are less prominent and arise more distally. The first diagonal branch tends to be the most prominent. When the first diagonal is large, the other diagonal vessels tend to be small and run a shorter course.
Right ventricular branches of the LAD, when present, are usually short and extend over the adjacent right ventricular surface, usually meeting right ventricular branches of the RCA. Occasionally, a prominent vessel (usually from the proximal LAD known as the left pre-infundibular artery) courses over the superior portion of the right ventricle to meet the conus artery and form the circle of Vieussens, as previously mentioned.
Interventricular branches, or septal perforating branches, descend from the LAD and travel down through the interventricular septum toward the smaller branches traveling upward from the posterior descending artery. These anterior septal perforators range in diameter from 0.5 to 1.2 mm and penetrate two thirds into the anterior septum. The length of these vessels ranges from 40 to 80 mm and tends to become shorter as they reach the apex. Like the septal branches of the posterior descending artery, the anterior septal perforators travel along the right ventricular border of the interventricular septum. The anterior septal perforators mechanically immobilize the LAD, fixing it to the heart, limiting its motion, and preventing buckling of the artery during systole.
Left circumflex artery
The left circumflex artery arises from the left main artery at almost a right angle. Its course nearly mirrors that of the RCA as it travels under the left atrial appendage, in the left AV sulcus, around the left acute margin, and toward the crux (Figures 1 and 7). Occasionally, the left circumflex artery may not course in the AV sulcus but rather descends over the left ventricular surface diagonally in the direction of the apex, terminating at the mid portion of the posterior interventricular sulcus. At its origin, the left circumflex artery has a diameter ranging between 1.5 and 5 mm. The degree of variability of the left circumflex artery and its branches is comparable to that of the RCA. At the crux, the left circumflex artery may extend to become the posterior descending artery and supply the AV node or may terminate, depending on dominance, as described above. Clinicians divide the circumflex into 3 branches: proximal (from vessel ostium to first major obtuse marginal branch), mid (between obtuse marginal one and two), and distal (vessel distal to the second obtuse marginal).
The left atrial branches of the left circumflex artery are named based on their origin: left anterior, marginal, or posterior. In 40% of subjects, the sinoatrial node is supplied from an atrial branch of the left circumflex artery. When this is the case, this branch travels upward along the left atrium, behind the aorta to the anterior interatrial sulcus, and continues rightward to partially encircle the lower portion of the superior vena cava. Kugel 10 described an early anterior atrial branch of the left circumflex artery. This artery has been termed arteria anastomotica auricularas magna, or Kugel's artery. There are 3 variations of this artery described. Likely, this artery represents an interatrial and atrial ventricular anastomotic network between the right coronary and left circumflex arteries.
The ventricular branches of the left circumflex artery branch at acute angles. From an anatomic standpoint, these arteries are named based on the segment of circumflex from which they arise: anterior, marginal, or posterior. In 80% of subjects, 1 to 3 anterior left ventricular branches are present. The number of posterior branches is variable and depends on the length of the left circumflex artery. In 80% of subjects, a left marginal (obtuse marginal) branch is present and is usually a large branch vessel with 2 or 3 secondary branches. Clinicians usually name the left ventricular branches of the circumflex artery obtuse marginals (OM1, OM2, OM3) (Figure 1A).
Anatomic variations
Isolated congenital coronary artery anomalies have been described in approximately 1% of patients who undergo coronary angiography 11 and approximately 0.3% of patients at autopsy. 12 Coronary artery anomalies may be classified as anomalies and variations without a shunt, anomalies with a shunt, or congenital aneurysms (Table 1). Anomalies and variations without a shunt include variations in coronary artery number, origin of vessel ostia, myocardial bridging, segmental hypoplasia, stenosis, or atresia. Anomalies with a shunt include coronary artery fistulas and coronary arteries originating from the pulmonary artery. Aneurysms of the coronary arteries may be congenital or acquired as a result of other disease processes. 13
Multislice ECG-gated cardiac CT is rapidly emerging as a useful noninvasive tool for the evaluation of the coronary arterial tree. The application of this tool that draws the most attention is screening for coronary artery stenoses. While the sensitivity for detection of coronary artery disease (80% to 95%) in the proximal arterial tree is very promising, 14 MSCT may be superior to cine arteriography in defining the ostial origin, proximal course, and termination of the coronary arteries and may be the "gold standard" for detecting coronary anomalies.
Although coronary anomalies are far less common than atherosclerosis, their impact on premature cardiac morbidity and mortality in young individuals needs to be emphasized. While some of these anomalies are benign and have no clinical sequela, others are associated with myocardial ischemia, ventricular dysfunction, and sudden death. 15,16 In a study by Eckart et al, 17 of 126 nontraumatic sudden deaths in young adults, cardiac abnormality was found in 64 cases (51%), with coronary artery abnormalities being the most common cardiac abnormality (39 of 64 patients [61%]). It has been suggested by several authors that MSCT with retrospective ECG gating may be superior to conventional angiography in defining vessel number, ostial origins, adjacent structure, and vessel course of the anomalous branches. 18 In addition, some anomalies are associated with other congenital cardiac malformations and should be recognized prior to any corrective surgery. Datta et al 19 performed cardiac MSCT on 17 patients referred because of equivocal findings at cardiac catheterization. In each case, the origin of the anomalous coronary artery and its course in relation to the great vessels were unequivocally identified. Several other authors have shown cardiac MSCT to be superior to cine coronary art-eriography for demonstrating anomalies of origin, course, and adjacent structures clarifying the diagnosis and more effectively guiding patient management. 20-22
Anomalies without a shunt
Anomalies in coronary number
A single coronary artery occurs in 0.024% of people. It is usually benign, but may be associated with congenital heart disease, such as transposition of the Great Arteries, tetrology of Fallot, truncus arteriosus, and coronary artery fistula. 20 Despite the anomalous origin, the peripheral coronary artery distribution is usually normal. This entity can easily be mistaken for 2 separate ostia originating from the same sinus of Valsalva or for atresia of a coronary ostium. 23 In 25% of patients with a single coronary artery, a major branch crosses the infundibulum, which can cause chest pain, myocardial infarction, or sudden death but also has technical implications for the surgeon when exposing the heart, instituting extracorporeal circulation, or when performing a right ventriculotomy.
A third coronary artery most commonly occurs when the conus artery, rather than branching from the proximal right coronary artery, has a separate ostium from the aorta. This occurs in approx-imately 50% of patients. 24 In addition, a third coronary artery can be seen when the left anterior descending and the left circumflex arteries have separate ostia and no left main artery is present (Figure 9). Four coronary arteries can be present when 2 of these variations occur simultaneously or when 2 conus arteries, each with separate ostia, are present.
Anomalies of coronary ostia
The coronary ostia are usually centrally located in the sinus of Valsalva, as described above. Some anomalous origins of coronary arteries have already been discussed, such as the anomalous conus artery, separate ostia of the LAD and left circumflex arteries, and single coronary artery. However, there are several other important variants to discuss.
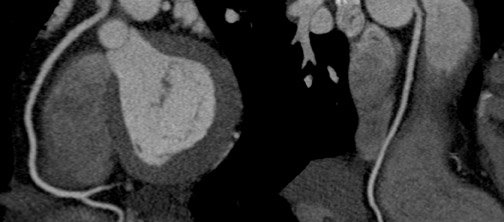
One or all of the coronary ostia may originate from the tubular portion of the aorta, above the sinotubular ridge. Vlodaver et al 25 reported that both coronary ostia were situated above the sinotubular ridge in 6% of randomly selected adult hearts. This becomes important to the operator attempting to perform coronary angiography, where selective intubation of the anomalous vessel may be extremely difficult, especially in the case of the right coronary artery with a high anterior ostium. When suspected and clinically indicated, MSCT can easily identify the anomalous origin of the vessel without the risk of invasive catheter-based methods (Figure 10).
Anomalous origin of the coronary artery (AOCA) from the opposite sinus of Valsalva is particularly important, as it has been associated with myocardial ischemia, ventricular arrhythmias, and sudden death, especially when the anomalous artery course is interarterial (between the aorta and the pulmonary artery). 15 Furthermore, when the anomalous vessel takes an interarterial course, it can travel in the myocardial sulcus between the great arteries (intramyocardial) or can travel within the anterior wall of the aorta (intramural). Anomalous origin of the left coronary from the right sinus of Valsalva with an interarterial course is rare (0.03% to 0.05%) 11 but is frequently associated with sudden cardiac death (Figure 19,26,27 Anomalous origin of the right coronary from the left sinus is more common (0.1%) 11 and is also associated with sudden cardiac death (Figure 12). 28,29 Anomalous origin of the circumflex is noteworthy, as the artery often takes a retroaortic course (Figure 13). This has surgical implications since it may be damaged by sutures placed in the mitral annulus during valve replacement or annuloplasty. 30 An AOCA from the noncoronary cusp is rare and is not associated with sudden death.
In the setting of AOCA, myocardial ischemia and sudden death tend to occur in young individuals and are often associated with extreme exertion, such as in competitive athletics. Very often, the patient will have no prior symptoms or signs of myocardial ischemia. Several theories have been proposed as possible mechanisms leading to myocardial ischemia. First, the ostium of the anomalous vessel is frequently slit-like and likely compromises flow. Secondly, the vessel usually arises from the aorta at an acute angle, rather than perpendicularly, which may alter the flow profile. Finally, it has been suggested that the interarterial course places the anomalous vessel at risk of compression between the great arteries. However, compression between the great vessels is less likely, as pressure within the pulmonary artery, even with strenuous exercise, is not high enough to compress and cause the collapse of a systemic vessel. It is more likely that during exercise, systemic arterial pressure rises, deforming the anomalous vessel within the aortic wall, causing flow disruption. This is more likely in patients with an intramural interarterial anomalous artery. Wall tension is proportional to the radius of the vessel. Therefore, the larger aorta will have greater wall tension than the much smaller coronary artery. As arterial pressure increases, aortic wall tension will outweigh coronary artery wall tension. This, theoretically, could flatten the intramural interarterial anomalous coronary artery, causing disruption of flow and myocardial ischemia. Zemanek et al 21 showed that, when compared with traditional arteriography, MSCT more accurately depicted coronary anatomy and more clearly revealed the interarterial course of the anomalous artery. This information has important diagnostic, prognostic, and therapeutic implications.
In patients who have myocardial ischemia, surgical repair is sometimes necessary. Several techniques have been attempted, including coronary bypass grafting, patch enlargement of the anomalous coronary, excision and reimplantation of the anomalous vessel to the correct sinus of Valsalva, or unroofing of the intramural portion of the anomalous segment. Unroofing of the vessel seems to be the most promising but is suited only for patients with an intramural interarterial anomalous vessel.
Myocardial bridge
During their course, coronary arteries (usually the LAD) are often covered by superficial muscle fibers, which run at right angles to the involved vessel, creating what is known as a "myocardial bridge." When present, only short portions of the vessel are covered by bridging fibers. On cine arteriograms, the bridged portion of the vessel can be visualized during systole, when the bridging fibers contract and distort the vessel lumen. Myocardial bridging has been associated with angina, myocardial infarction, and sudden death. Ironically, the bridged segment is rarely affected by atherosclerosis and can easily go unrecognized on cine arteriography as what otherwise appears to be a normal coronary artery. Multislice cardiac CT can detect the presence of myocardial bridging if suspected. However, the ECG-gated reconstruction window in multidetector MSCT of the coronary arteries is done during diastole, when the coronaries are maximally filled and have the least amount of motion. To recognize bridging, ECG-gated reconstruction must be done during systole and diastole. Rychter et al 31 presented a case series of patients with atypical angina in whom myocardial bridging was easily identified on cardiac MSCT (Figure 14).
Coronary hypoplasia
Isolated coronary hypoplasia, stenoses, or atresia are extremely uncommon. In ostial atresia of the left coronary artery, injection of contrast into the right coronary slowly fills the left coronary by collateral flow. The left coronary system is usually hypoplastic, and ischemia is frequent. Supravalvular aortic stenosis often causes pathologic changes in the coronary arteries. Stenosis or atresia of the coronary arteries in childhood may occur in association with a variety of vascular or systemic diseases, including coronary calcinosis, homocystinuria, progeria, mucopolysaccharidosis, Friedreich's ataxia, and Williams syndrome of supravalvular aortic stenosis.
Anomalies with a shunt
Coronary artery fistulas
Coronary artery fistulas are abnormal communications between a coronary artery and another vascular structure: an artery, vein, or cardiac chamber. This condition is seen in approximately 0.1% to 0.2% of all patients who undergo selective coronary angiography. 32 More often, the fistula is formed with a right sided (venous) structure. The most common sites of drainage are the right ventricle (45%), the right atrium (25%), and the pulmonary artery (15%), but fistulas to the superior vena cava and coronary sinus have been reported. Coronary artery fistulas to the left atrium and left ventricle are less common (<10% of cases). 33 The right coronary artery forms a fistula slightly more often than the left coronary artery (Figure 15). 34
The involved coronary artery is dilated and often tortuous because of increased flow. 35 Arteriography will show a dilated coronary artery with rapid flow and prompt opacification of the draining chamber. The normal arterial branches distal to the fistula may be small and poorly filled because of steal of flow from the fistula.
When the fistula occurs with a venous structure, a left-to-right shunt is created. The largest shunts occur when the connection is with the right atrium, which can cause significant hemodynamic derangements similar to that of atrial or ventricular septal defects. When the fistula forms with the left atrium or ventricle, the hemodynamic derangements that occur resemble that of aortic insufficiency. In addition, the region of myocardium normally supplied by the involved coronary artery may have diminished flow, creating a hemodynamic steal phenomenon, and can lead to myocardial ischemia. 36
Knowledge of coronary fistulas is important for prognosis and management. Surgical closure of congenital coronary fistulas in adults can be performed with a very low risk, and surgical closure is recommended to prevent complications. 37
Anomalous origin of the coronary artery from the pulmonary artery
Anomalous origin of the coronary artery from the pulmonary artery (ALCAPA) usually affects the left coronary artery but may involve the right, both arteries, or an accessory coronary branch. In the most common form, Bland-White-Garland syndrome, the left coronary artery arises from the pulmonary artery, and the right coronary artery arises normally from the aorta (Figure 16). 38 In this case, the left coronary artery courses adjacent to its normal aortic origin, near the left sinus, before branching in the normal left coronary distribution.
Since coronary perfusion of the myocardium occurs during diastole, flow is dependent on the diastolic pressure gradient between the coronary artery and the myocardial bed it perfuses. After birth, diastolic pulmonary artery pressure decreases and systemic arterial pressure increases. Therefore, diastolic perfusion pressure across the myocardial beds fall and ischemia occurs. Perfusion of the left coronary bed is retrograde from the right coronary.
This is a rare congenital anomaly, occurring in 1 in 300,000 children. Most affected patients have signs and symptoms during infancy. Approximately 90% of untreated infants die in the first year of life. 39
Congenital aneurysms
Congenital coronary artery aneurysms are indistinguishable from those acquired secondary to other disease processes. The aneurysms may be multiple and can appear saccular or fusiform. Major coronary aneurysms can rupture, thrombose, or produce infarction due to thromboembolism.
Conclusions
With the evolution of new and exciting modalities, such as multidetector ECG-gated cardiac MSCT, noninvasive imaging of small mobile structures, such as coronary arteries, has become possible. Because of the high prevalence, morbidity, mortality, and enormous socioeconomic burden of coronary artery disease, noninvasive detection of significant coronary artery stenoses has been the driving force behind the development of this technology. As the technology evolves, cardiac CT will become readily available, making interpretation a necessary clinical skill. To interpret cardiac CT, one must appreciate normal coronary anatomy, recognizing the normal origin, course, branching, and termination of coronary arteries with regard to adjacent structures. In addition, coronary anomalies can be easily visualized with this technology. Therefore, knowledge of the various coronary anomalies is important to those interpreting cardiac CT.