MR of articular cartilage lesions of the knee





Dr. Chang is a Radiology Resident and Dr. Huang is an Assistant Radiologist in the Division of Musculoskeletal Imaging and Intervention, Department of Radiology, Massachusetts General Hospital, Boston, MA. In addition, Dr. Huang is an Instructor of Radiology at Harvard Medical School, Boston, MA.
This article is based on “MR of articular cartilage lesions,” presented at Sports Medicine 2010: Advances in MRI and Orthopaedic Management, Department of Continuing Education, Harvard Medical School, Boston, MA, May 9, 2010.
Osteoarthritis is a highly prevalent disease in the United States population, with approximately 75% of persons over age 65 having radiographic evidence of degenerative changes, and nearly two-thirds of routine knee magnetic resonance imaging (MRI) demonstrating articular cartilage damage.1 Therefore, careful evaluation of the articular cartilage is an important part of the knee MRI examination.
Basic imaging principles
Routine 2-dimensional imaging of the knee includes fast spin-echo (FSE) T2- and/or FSE proton density (PD)-weighted coronal and sagittal sequences, with or without fat suppression. The contrast between the high T2 signal intensity joint fluid and the intermediate T2 signal intensity cartilage helps to assess for surface irregularities and defects of the cartilage.2 FSE PD-weighted images provide excellent anatomic detail and evaluation of all structures, including cartilage, menisci, ligaments, and subchondral bone.3,4 Fat suppression helps to highlight marrow edema and cystic changes underlying cartilage lesions and to eliminate chemical shift artifact seen at the marrow (fat)-cartilage (water) interfaces.5
The 3-dimensional spoiled gradient echo (SPGR) sequence with fat suppression yields higher cartilage signal and spatial resolution and is therefore useful for cartilage thickness and volume measurements. However, 3-dimensional SPGR is not typically included in imaging protocols because of decreased contrast between the cartilage and the adjacent joint fluid, long imaging times, increased metallic artifact (in the case of a post-surgical knee), and uneven fat suppression, even though it is considered standard for morphologic imaging of cartilage.6
At least one sequence in both sagittal and coronal planes is necessary to prevent overcalling of lesions due to volume averaging in the direction perpendicular to the slice plane because while in-plane resolution may be 0.3 mm to 0.6 mm, slice thickness is typically 3 mm to 5 mm. Table 1 lists several MR cartilage imaging pitfalls and troubleshooting techniques.7,8
MR imaging at 3.0 Tesla (T) versus 1.5 T improves signal-to-noise ratio, lesion detection, and grade assessment.9-11 Various other techniques have been developed to enhance the signal-to-noise ratio between cartilage and its surrounding structures,12,13 and to evaluate the molecular changes in the cartilage to detect early cartilage injury6, 14-21 but these techniques are not routinely utilized. MR arthrography (MRA) of the knee also helps to increase sensitivity for cartilage lesions and for detection of intra-articular bodies and their donor sites, but it is also not routinely performed. Indications for MRA include evaluation of osteochondral defects for stability and evaluation of postoperative menisci for retear.22-24
Normal appearance and cartilage lesions
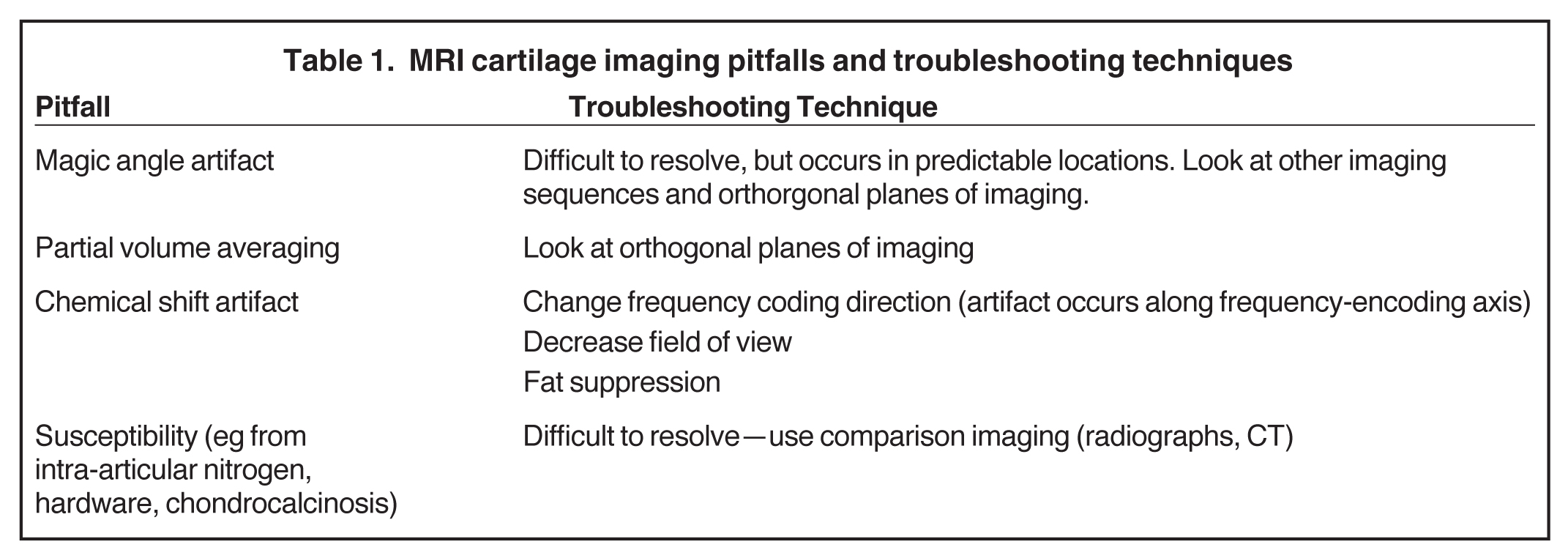
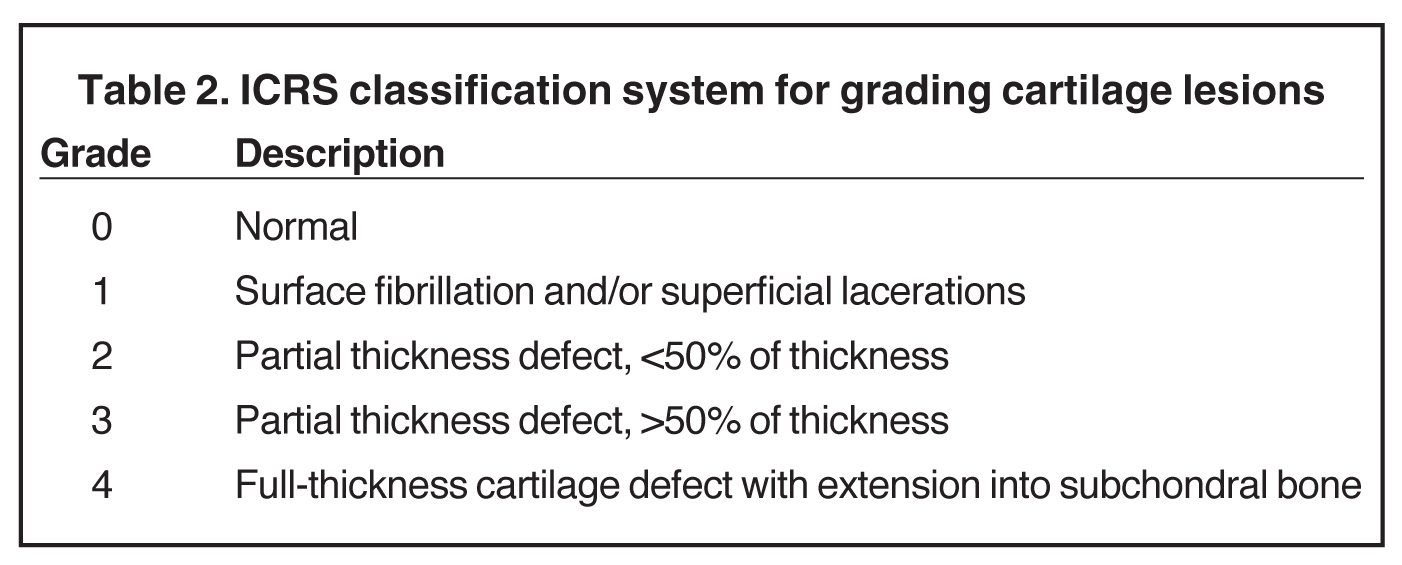
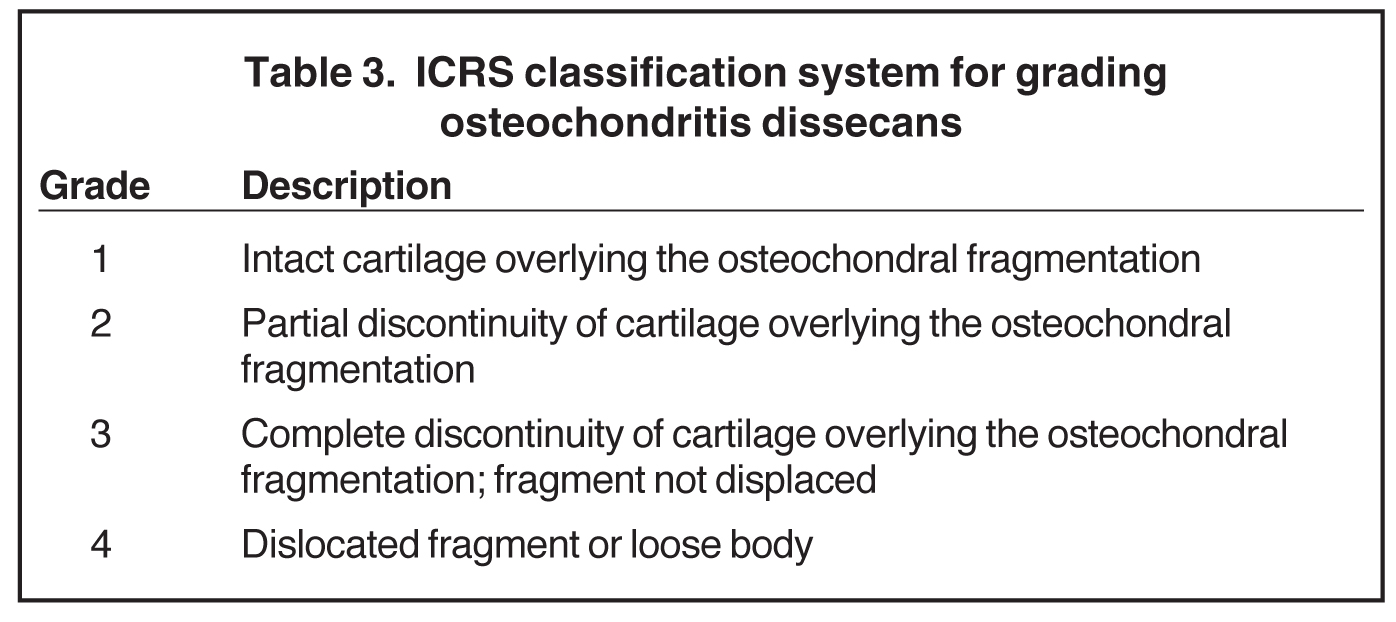
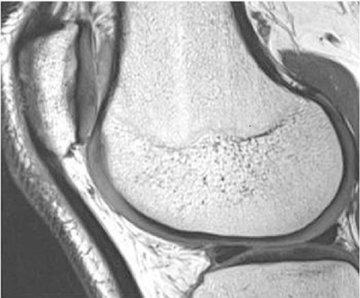
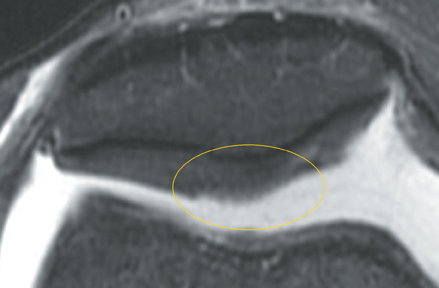
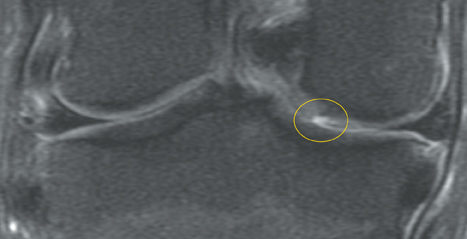
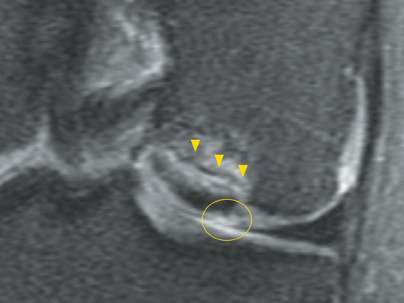
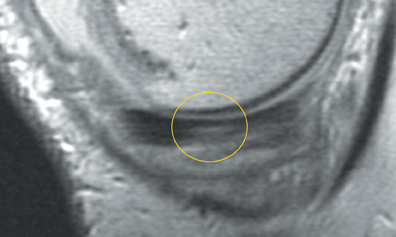
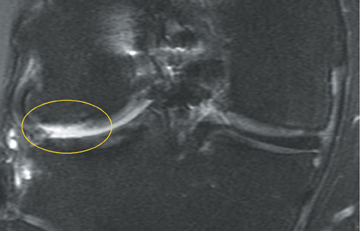
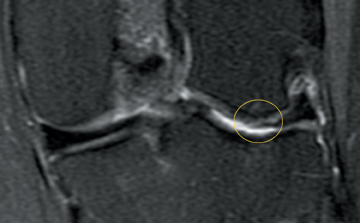
Cartilage is approximately 75% to 80% water by weight and therefore appears slightly less hyperintense to joint fluid on T2-weighted images.21 Matrix collagen has a leaf-like structure with proteoglycans projecting from a central hyaluronic acid backbone. The collagen leaves extend perpendicularly from the bony surface and curve 90 degrees until they are approximately horizontal or parallel to the bone at the articular surface. The different angles and orientations of the proteoglycans likely affect the water mobility within and the dipole-dipole interactions of the collagen fibrils, which result in the MR appearance of cartilage on a T2-weighted sequence as three poorly demarcated layers: a) a low signal intensity, striated, deep layer (perpendicular portion, ≈50% of thickness); b) a high signal intensity transitional layer (curving portion, ≈40% of thickness); and c) a low signal intensity superficial layer (horizontal portion, ≈10% of thickness) (Figure 1). The orientation of the cartilage relative to the orientation of the static magnetic field (B0) also affects the thickness, signal intensity, and distinctness of these layers. Therefore, since much of the cartilage surfaces of the knee are curved (eg, the femoral condyles), appearance of the articular cartilage varies. The uniformity of the collagen fiber orientation at the articular and the bony surfaces helps to elucidate the reason magic angle phenomenon affects cartilage (Figure 2), and the curvature of the collagen fibers also helps to explain the curved appearance of many cartilage lesions.5,25-27
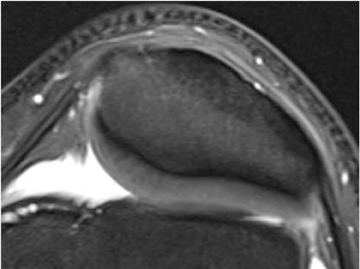
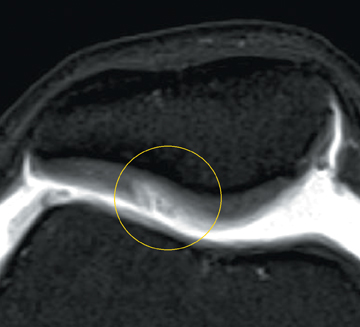
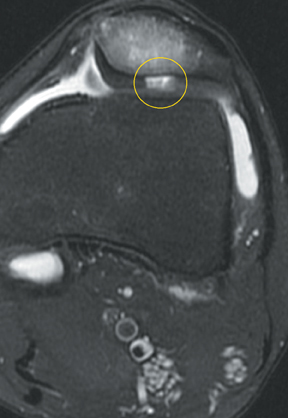
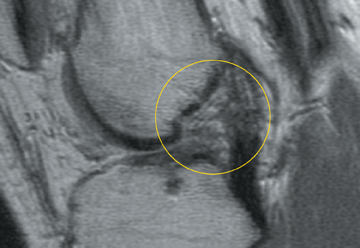
Chondromalacia, or cartilage softening, without surface cartilage defect is the earliest stage of cartilage injury and may appear on MRI as focal areas of increased signal intensity on T2-weighted images (Figure 3). This finding is nonspecific, as it is often seen in asymptomatic patients; concurrent underlying marrow signal abnormality increases its specificity.7,28,29
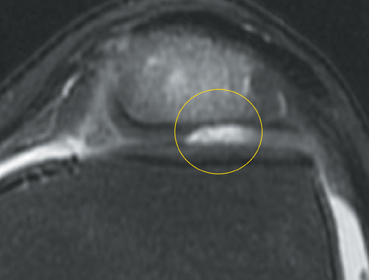
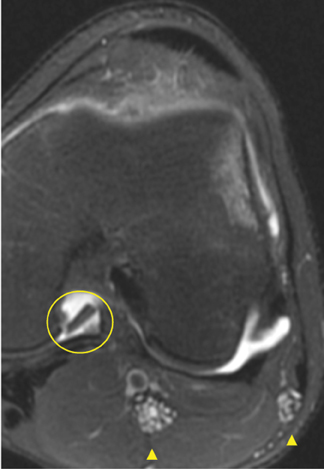
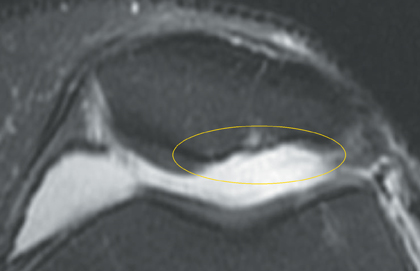
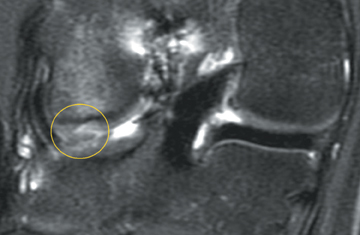
Cartilage defects appear as synovial fluid-filled, T2 hyperintense gaps that disrupt the articular surface. The International Cartilage Repair Society (ICRS) has standardized the evaluation of articular cartilage injury based on the depth of the lesion. The lesion can be described as a superficial defect or surface irregularity (Figure 4), partial-thickness defect (Figure 5), or full-thickness defect (Figure 6). If spatial resolution allows, it may be possible to further subdivide partial thickness defects into low-grade and high-grade partial thickness defects depending on whether the lesion involves less than 50% or 50% to 99% of the full cartilage thickness. Partial and full-thickness lesions may be associated with flap formation (Figure 7).
Numerous classification schemes exist for grading cartilage lesions, most of which take into account the size (area) and/or depth of the lesion. The ICRS has developed one such system, in which lesions are graded 0 to 4 based on depth of the lesion (Table 2). Grade 1 and 2 lesions have excellent prognosis. Grade 2 and 3 lesions may benefit from cartilage debridement or other more conservative surgical measures. Grade 4 lesions extend into the subchondral bone and may require bone grafting if bony cavitation is extensive. Subchondral marrow edema is more likely to be associated with higher-grade lesions30 and, in the setting of acute trauma, indicates that the lesion may be full thickness.
Since the grading of cartilage lesions is largely a matter of local practice preference and no one classification scheme is overwhelmingly used over all others, our practice reports the size, depth, and location of the cartilage lesion to allow the referring physicians to fit the lesion into their own grading system.
MRI has been shown to be an accurate method for detection of cartilage lesions. Bredella et al2 reported that the sensitivity, specificity, and accuracy of combined axial and coronal FSE T2-weighted sequences with fat suppression compared with the gold standard of arthroscopy were 94%, 99%, and 98%, respectively. In their study, 64% of the lesions were given the same grade as that shown during arthroscopy. Ninety percent of lesions were within one grade between MRI and arthroscopy, and the majority of the lesions undergraded on MR imaging. Potter et al4 reported that the sensitivity, specificity, and accuracy of FSE PD-weighted sequences compared with arthroscopy were 87%, 94% to 95%, and 92% to 93%, respectively. In general, MRI underestimates the true dimensions of a cartilage defect due to volume averaging and challenges of imaging a curved surface.31
Cartilage disease can be separated into 3 broad categories: acute chondral or osteochondral injury, osteoarthritis, and inflammatory arthritis. There are some distinguishing characteristics of each category. An acute lesion has sharp margins oriented perpendicular to the bone surface, occurs on weight-bearing surfaces, and has subjacent bone marrow edema (Figure 8).23,26 The fractured cartilage may break off as a “loose” fragment; searching for these intra-articular bodies on knee MRI in acute chondral injury is imperative because of the implications for surgery.
In osteoarthritis, early proteoglycan loss leads to expansion of the residual proteoglycans and increased cartilage water content, which may appear as increased cartilage thickness on MRI. This edematous cartilage deforms more easily and is therefore more susceptible to mechanical stress and cartilage damage. Eventually, the cartilage matrix fragments. Cartilage lesions in osteoarthritis tend to be shallower and have wide, horizontal, or obtuse margins with respect to the bone surface (Figure 9). Because synovial fluid is toxic to bone marrow, subchondral cysts form if the defect extends to the subchondral bone surface. Lesions typically occur in the weight-bearing portions of the joint and result in nonuniform joint space narrowing, one of the hallmarks of osteoarthritis. Chondrocalcinosis in osteoarthritis indicates attempts at repair in response to a prior insult.22,32,33
In inflammatory arthritis, cartilage loss is more diffuse and uniform. The enzymes produced by the pannus cause cartilage breakdown. The pannus also forms a physical barrier for diffusion of nutrients to chondrocytes, further promoting chondrocyte death. Focal lesions are less common. Inflammatory changes of the subchondral bone and chondrocalcinosis are also seen.26
In osteochondritis dissecans (OCD), the primary role of MRI is to determine stability of the defect. Findings suggestive of instability include torn overlying articular cartilage, undercutting of osteochondral fragment with fluid signal, osteochondral defect with fluid-filled cavity, and cystic change (5 mm or larger) deep to the lesion (Figure 10). The ICRS has developed a grading system for OCD lesions (Table 3), but it requires arthroscopic probing for definitive lesion classification. However, lesions found to be stable by MRI have been found to have a good clinical outcome, while articular surface defects detected by MRI predict a poor clinical outcome.34
Factors leading to accelerated cartilage damage
The cartilage, menisci, ligaments, and bony structures in the knee all contribute to distribution of the load forces in the knee. Therefore, abnormalities of any one of those structures invariably leads to the early degeneration of the other structures.27
Meniscal tear
Preoperative detection of a cartilage flap associated with a meniscal tear is important in presurgical planning for the surgeon and modulating expectations for length of rehabilitation time for the patient.35 Cartilage loss following menisecectomy may be accelerated (Figure 11); therefore, meniscal debridement has largely replaced total meniscectomy in the surgical management of meniscal tears to preserve stability and function and to delay the onset of osteoarthritis.20,36 MRI is helpful in imaging the postoperative knee because it can be clinically difficult to distinguish between a meniscal retear, a chondral lesion, or an osseous lesion as the source of symptoms in the post-meniscectomy knee.
ACL tear
The mechanism of injury in anterior cruciate ligament (ACL) ruptures includes significant axial loading. The articular cartilage transmits these forces, and this initial impact results in cartilage changes that often lead to accelerated osteoarthritis, despite immediate stabilization of the knee with ACL reconstruction (Figure 12). The sulcus terminalis on the lateral femoral condyle and the posterolateral tibial plateau—also the location of the “kissing contusions” associated with ACL tears—are the most common locations for these cartilage lesions.
Early cartilage injury and chondromalacia may or may not be visible on visual inspection during arthroscopy but can be detected through probing during arthroscopy and can be seen as increased signal intensity on T2-weighted images. More severe trauma or chronic repetitive trauma may result in an osteochondral lesion, which appears as the “deep lateral femoral notch sign” at the sulcus terminalis.35,37
Cartilage surgery
Advances in cartilage surgery include bone marrow stimulation through microfracture, osteochondral autograft or allograft transplantation, autologous chondrocyte implantation, and fixation with bio-absorbable pins. Regardless of the type of procedure being performed, routine MRI after cartilage surgery should address these concerns:
- Graft quality. The graft ideally should fill in the defect and be level with the articular surface. The signal intensity of the graft should match the signal intensity of the adjacent native cartilage. A failed graft may appear fragmented, demonstrate surface irregularity, or be resorbed.
- Graft incorporation. A well-incorporated graft will be practically seamless with the adjacent cartilage. A poorly incorporated graft may demonstrate persistent fluid signal separating the graft and the bone, new subchondral cyst formation, and/or new adjacent sclerosis.
- Native cartilage quality. Accelerated osteoarthritis, including cartilage loss and other cartilage lesions, may form as the consequence of altered mechanics from the primary cartilage injury.
- Other. Joint synovitis or adhesions near the repair site may cause the patient symptoms unrelated to the cartilage graft itself.
By approximately 1 to 2 years following surgery, the defect should appear smooth and well-filled by the reparative tissue (Figure 13).24,38
Future directions
Physiologic imaging aims to detect cartilage lesions before they are visible as surface defects. For example, delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) identifies areas of decreased cartilage proteoglycan content.15 T2-mapping detects areas of increased tissue hydration within cartilage.39 Other developing techniques include T1ρ mapping, sodium MRI, and diffusion imaging, all of which take advantage of the physiologic properties of cartilage to show microscopic changes in the cartilage ultrastructure before gross damage to the cartilage surface is apparent. While for most patients the morphologic imaging sequences may suffice, physiologic imaging may be useful for more detailed or longitudinal evaluation of surgical procedures (ie, cartilage implants, medial meniscal repair for prevention of osteoarthritis progression) or pharmacologic therapies.6,15,21,35
Conclusion
Cartilage lesions are very commonly seen on knee MRI imaging. Current imaging techniques accurately diagnose and grade cartilage lesions, and developing techniques for physiologic imaging show promise for longitudinal evaluation of normal, degenerated, and postsurgical cartilage. As medical and surgical therapies improve, MRI will play an increasing role in diagnosis and management of knee cartilage lesions, and familiarity with lesion terminology and accurate descriptions of cartilage lesions will enhance communication between radiologists and referring physicians.
References
- Disler DG, McCauley TR, Kelman CG, et al. Fat-suppressed three-dimensional spoiled gradient-echo MR imaging of hyaline cartilage defects in the knee: Comparison with standard MR imaging and arthroscopy. AJR Am J Roentgenol. 1996;167:127-132.
- Bredella MA, Tirman PF, Peterfy CG, et al. Accuracy of T2-weighted fast spin-echo MR imaging with fat saturation in detecting cartilage defects in the knee: Comparison with arthroscopy in 130 patients. AJR Am J Roentgenol. 1999;172:1073-1080.
- Sonin AH, Pensy RA, Mulligan ME, Hatem S. Grading articular cartilage of the knee using fast spin-echo proton density-weighted MR imaging without fat suppression. AJR Am J Roentgenol. 2002;179:1159-1166.
- Potter HG, Linklater JM, Allen AA, et al. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80:1276-1284.
- Disler DG, McCauley TR. Clinical magnetic resonance imaging of articular cartilage. Top Magn Reson Imaging. 1998;9:360-376.
- Gold GE, Hargreaves BA, Stevens KJ, Beaulieu CF. Advanced magnetic resonance imaging of articular cartilage. Orthop Clin North Am. 2006;37:331-347, vi.
- Hayes CW, Conway WF. Evaluation of articular cartilage: Radiographic and cross-sectional imaging techniques. Radiographics. 1992;12:409-428.
- Waldschmidt JG, Rilling RJ, Kajdacsy-Balla AA, et al. In vitro and in vivo MR imaging of hyaline cartilage: Zonal anatomy, imaging pitfalls, and pathologic conditions. Radiographics. 1997;17:1387-1402.
- Masi JN, Sell CA, Phan C, et al. Cartilage MR imaging at 3.0 versus that at 1.5 T: Preliminary results in a porcine model. Radiology. 2005;236:140-150.
- Link TM, Sell CA, Masi JN, et al. 3.0 vs 1.5 T MRI in the detection of focal cartilage pathology--ROC analysis in an experimental model. Osteoarthritis Cartilage. 2006;14:63-70.
- Kijowski R, Blankenbaker DG, Davis KW, et al. Comparison of 1.5- and 3.0-T MR imaging for evaluating the articular cartilage of the knee joint. Radiology. 2009;250:839-848.
- Hargreaves BA, Gold GE, Lang PK, et al. MR imaging of articular cartilage using driven equilibrium. Magn Reson Med. 1999;42:695-703.
- Hargreaves BA, Gold GE, Beaulieu CF, et al. Comparison of new sequences for high-resolution cartilage imaging. Magn Reson Med. 2003;49:700-709.
- Gold GE, McCauley TR, Gray ML, Disler DG. What’s new in cartilage? Radiographics. 2003;23: 1227-1242.
- Burstein D, Velyvis J, Scott KT, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45:36-41.
- Lammentausta E, Kiviranta P, Nissi MJ, et al. T2 relaxation time and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) of human patellar cartilage at 1.5 T and 9.4 T: Relationships with tissue mechanical properties. J Orthop Res. 2006;24: 366-374.
- Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41:857-865.
- Reddy R, Insko EK, Noyszewski EA, et al. Sodium MRI of human articular cartilage in vivo. Magn Reson Med. 1998;39:697-701.
- Recht M, Bobic V, Burstein D, et al. Magnetic resonance imaging of articular cartilage. Clin Orthop Relat Res. 2001;(391 Suppl):S379-S396.
- Recht MP, Kramer J. MR imaging of the postoperative knee: A pictorial essay. Radiographics. 2002;22:765-774.
- Van Breuseghem I. Ultrastructural MR imaging techniques of the knee articular cartilage: Problems for routine clinical application. Eur Radiol. 2004;14:184-192.
- Gagliardi JA, Chung EM, Chandnani VP, et al. Detection and staging of chondromalacia patellae: Relative efficacies of conventional MR imaging, MR arthrography, and CT arthrography. AJR Am J Roentgenol. 1994;163:629-636.
- Chung CB, Isaza IL, Angulo M, et al. MR arthrography of the knee: How, why, when. Radiol Clin North Am. 2005;43:733-746, viii-ix.
- Miller TT. MR imaging of the knee. Sports Med Arthrosc. 2009;17:56-67.
- Goodwin DW, Zhu H, Dunn JF. In vitro MR imaging of hyaline cartilage: Correlation with scanning electron microscopy. AJR Am J Roentgenol. 2000;174:405-409.
- Jeffrey DR, Watt I. Imaging hyaline cartilage. Br J Radiol. 2003;76:777-787.
- Burstein D, Bashir A, Gray ML. MRI techniques in early stages of cartilage disease. Invest Radiol. 2000;35:622-638.
- Rose PM, Demlow TA, Szumowski J, Quinn SF. Chondromalacia patellae: Fat-suppressed MR imaging. Radiology. 1994;193:437-440.
- Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: Influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology. 2000;214:259-266.
- Kijowski R, Stanton P, Fine J, DeSmet A. Subchondral bone marrow edema in patients with degeneration of the articular cartilage of the knee joint. Radiology. 2006;238:943-949.
- Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A Suppl 2:58-69.
- Dardzinski BJ, Mosher TJ, Li S,et al. Spatial variation of T2 in human articular cartilage. Radiology. 1997;205:546-550.
- Kleemann RU, Krocker D, Cedraro A, et al. Altered cartilage mechanics and histology in knee osteoarthritis: Relation to clinical assessment (ICRS Grade). Osteoarthritis Cartilage. 2005;13:958-963.
- De Smet AA, Ilahi OA, Graf BK. Untreated osteochondritis dissecans of the femoral condyles: Prediction of patient outcome using radiographic and MR findings. Skeletal Radiol. 1997;26:463-467.
- Potter HG, Foo LF. Magnetic resonance imaging of articular cartilage: Trauma, degeneration, and repair. Am J Sports Med. 2006;34:661-677.
- Cicuttini FM, Forbes A, Yuanyuan W, et al. Rate of knee cartilage loss after partial meniscectomy. J Rheumatol. 2002;29:1954-1956.
- Johnson DL, Urban WP, Caborn DN, et al. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med. 1998;26:409-414.
- Choi YS, Potter HG, Chun TJ. MR imaging of cartilage repair in the knee and ankle. Radiographics. 2008;28:1043-1059.
- Kelly BT, Potter HG, Deng XH, et al. Meniscal allograft transplantation in the sheep knee: Evaluation of chondroprotective effects. Am J Sports Med. 2006;34:1464-1477.
