MR Imaging of pericardial diseases
Images

The pericardium can be affected by infectious or inflammatory processes causing pericarditis; accumulation of excess fluid, causing tamponade, thickening and scarring of the layers leading to constriction; primary and metastatic neoplasms, and congenital processes that can be mistaken for masses or predispose to acute cardiac decompensation. In most cases, clinical examination and echocardiography are sufficient for diagnosis and management. However, subtle or overlapping clinical presentations and limited echocardiographic windows can occasionally lead to diagnostic uncertainty. Cardiac magnetic resonance (CMR) imaging has emerged as a powerful tool in the evaluation of pericardial disease. This article focuses on the MR characteristics of the most commonly encountered pericardial diseases and the adjunct role CMR can play in patient evaluation and management.
Normal CMR appearance of the pericardium
The pericardium envelopes the heart and provides a relatively frictionless environment for cardiac contraction and an impediment to contiguous spread of infection or disease to the myocardium. The pericardium consists of a thick, fibrous parietal layer and a thinner visceral layer, the latter of which closely approximates the epicardial fat and—under normal circumstances—is too thin to be routinely visualized at CMR. The pericardium typically contains less than 50 mL of normal serous fluid, most of which resides in various pericardial recesses and the oblique and transverse pericardial sinuses which are formed by reflections of the serous pericardial layer.1 The normal pericardial thickness is approximately 2 mm, although a thickness of up to 4 mm is not necessarily abnormal.2
Normal pericardium shows low signal intensity on all pulse sequences. Owing to its contrast with the surrounding fat, the pericardium is best delineated anterior to the right ventricle. Pericardium inferoposterior to the left ventricle is often poorly visualized due to the paucity of adjacent fat. In addition, pericardial visibility is better on caudal sections of the heart because the pericardium is thicker in this region due to its insertion onto the diaphragm. Normal serous pericardial fluid will demonstrate high signal intensity on cine steady state free precession (SSFP) sequences. Thus, physiological pericardial fluid can be readily distinguished from thickened pericardium by means of cine acquisitions. On T2-weighted black-blood sequences, normal pericardial fluid is expected to have high signal intensity, but its cardiac related non-laminar motion may change the signal intensity with this artifact more pronounced in small versus large pericardial effusions. In general, fat suppression sequences limit the ability to distinguish between pericardium and pericardial fluid.
Acute pericarditis and pericardial tamponade
Many, but not all, patients with acute pericarditis will present with acute onset of chest pain that is characteristically positional in nature, being relieved when sitting up or leaning forward, and is often preceded by a viral-like prodrome. ST segment elevations can mimic acute myocardial infarction, leading some patients to undergo catheter angiography. On physical examination, a pericardial friction rub can be auscultated in many patients. Echocardiography is the most appropriate first line imaging modality and may demonstrate a pericardial effusion. Clinical criteria for the diagnosis of acute pericarditis require 2 of the 4 following findings: typical chest pain, typical EKG changes, pericardial friction rub and new or worsening pericardial effusion. 3
In the U.S., most cases of acute pericarditis are idiopathic, with many presumed to be viral in etiology.4 Bacterial infections are rare and more commonly encountered in postoperative cardiac patients and immunocompromised individuals. However, worldwide and in underdeveloped countries, tuberculosis remains a common cause of pericarditis. Numerous noninfectious causes are known as well including mediastinal radiation, connective tissue diseases and myocardial infarction.5
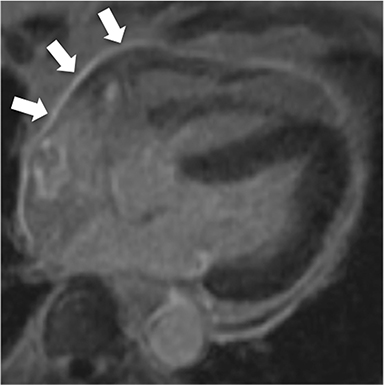
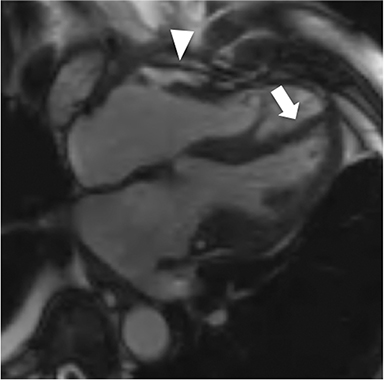
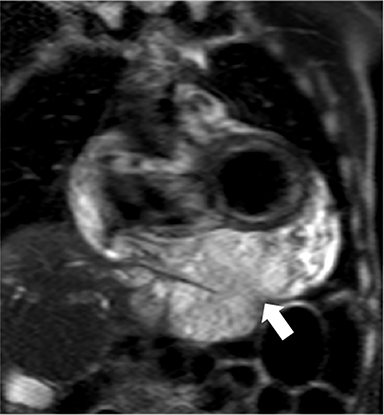
At CMR, thickening of the pericardium to greater than 4 mm can be seen, with the pericardium often best demonstrated on non-fat-suppressed SE or turbo SE sequences, where it is low in signal intensity and outlined by epicardial and pericardial fat. T2-weighted, short tau inversion recovery (STIR) sequences can demonstrate edema within and surrounding the pericardium in addition to demonstrating the amount and location of pericardial fluid.6 As most cases have transudative pericardial effusions, the signal intensity of an effusion is low on T1-weighted sequences and high on T2-weighted sequences. In the case of hemorrhagic or suppurative effusions, the T1 signal is often increased and varying degrees of T2 hyperintensity are seen. Myocardial-ed late gadolinium enhanced (LGE) sequences nicely demonstrate pericardial enhancement (Figure 1).7 CMR can also detect associated myocarditis (so called “myopericarditis”) by the finding of edema and delayed enhancement in subepicardial or mesocardial distributions and, if present, can quantify any ventricular functional impairment.
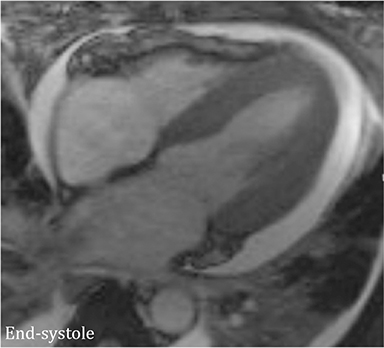
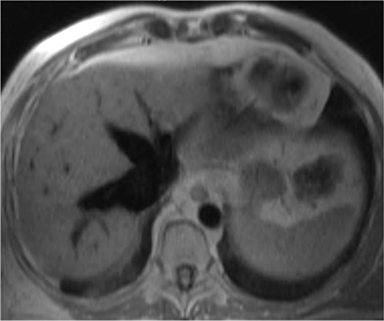
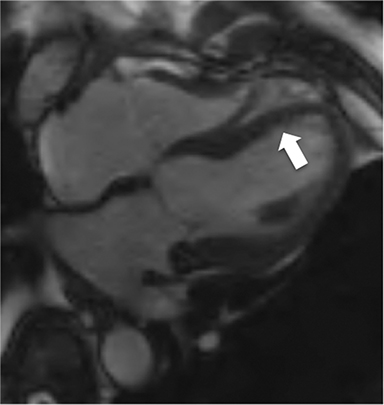
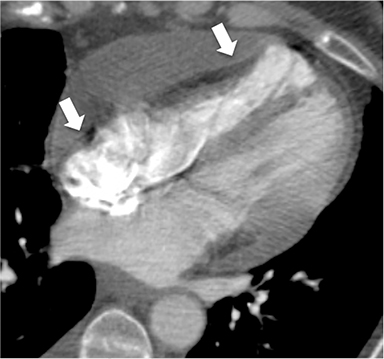
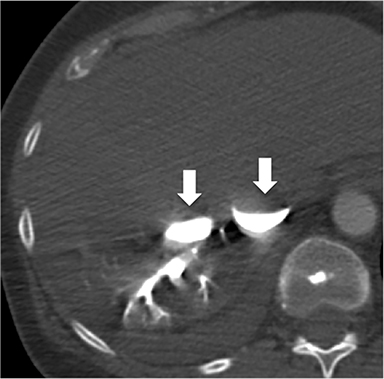
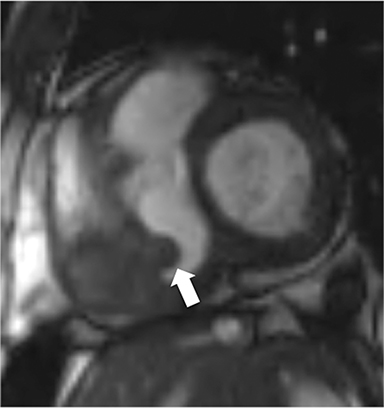
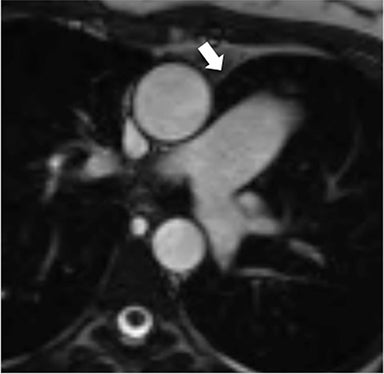
The accumulation of excess pericardial fluid can lead to impairment of diastolic filling and subsequently cardiac output known as cardiac tamponade. Only a small amount of fluid is required in the acute setting to exceed ventricular diastolic pressures; however, in chronic pericardial effusions/inflammation the gradual accumulation over time allows for pericardial distention allowing more fluid to develop before tamponade occurs. Whenever a pericardial effusion is detected at CMR, assessment for signs of tamponade should occur which include early diastolic flattening of the right ventricular free wall, late diastolic and early systolic right atrial notching present during greater than one third of the cardiac cycle, and dilatation of the superior and inferior vena cava (Figure 2).8,9
The vast majority of acute pericarditis resolves with conservative management (non-steroidal anti-inflammatory drugs with or without colchicine). Occasionally, chronic (>3 months) or recurrent pericarditis may develop. Patients with suppurative or tuberculous pericarditis are at higher risk for developing constrictive pericarditis. Patients with large pericardial effusions or cardiac tamponade undergo pericardiocentesis and may require drain placement or pericardial window to prevent recurrence.
Constrictive pericarditis
When the pericardium becomes thickened or fibrosed, it becomes less compliant and prevents adequate diastolic filling of the ventricles, a condition known as constrictive pericarditis (CP). In the US and most other developed countries, the most common causes are prior surgical violation of the pericardium, mediastinal radiation and the sequela of prior infectious (viral) pericarditis. Worldwide, tuberculous pericarditis has a high propensity to progress to CP.3
Due to the inherently lower intracardiac pressures of the right heart, the effects of pericardial constriction are first manifest as right heart failure with patients commonly presenting with lower extremity swelling, liver dysfunction, and dyspnea on exertion. While echocardiography remains the first line evaluation for CP, both CT and MR have emerged as important adjunct imaging modalities to clarify both the extent and physiologic effects of diseased pericardium. CMR also plays an important role in distinguishing between CP and restrictive cardiomyopathy, two entities whose signs and symptoms overlap but whose management strategies are quite different. CP is treated surgically with pericardial stripping, while restrictive disease is managed medically.
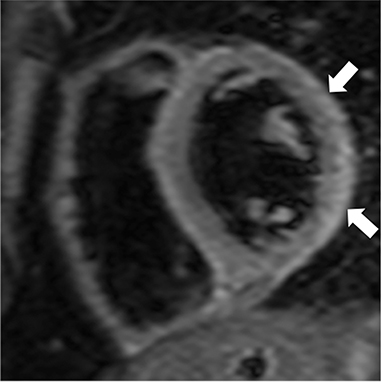
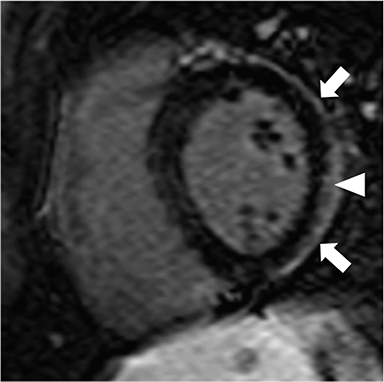
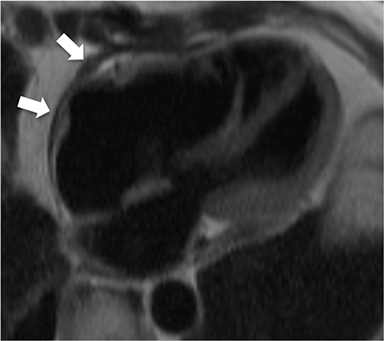
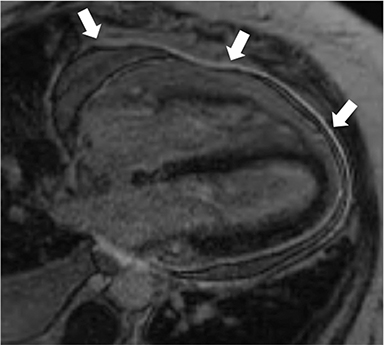
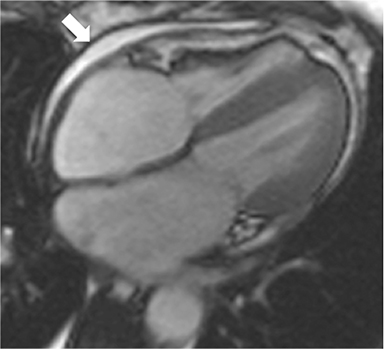
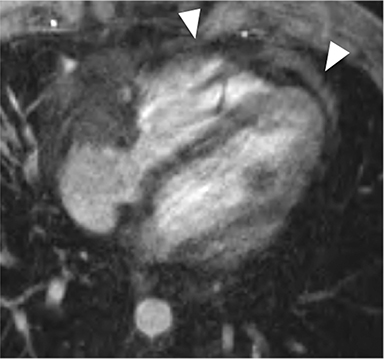
Non-fat suppressed T1 and T2 turbo SE sequences in multiple planes are useful to adequately visualize the entirety of the low signal intensity pericardium. Normal pericardial thickness is less than 2mm with greater than 4mm of thickening considered abnormal. In the appropriate clinical context, the finding of pericardial thickening greater than 4mm is suggestive of CP and thickening greater than 6mm in considered highly specific1 (Figure 3). However, it is known than up to 20% of patients with CP will have normal pericardial thickness; therefore, the absence of thickening does not exclude its presence.4,10 Pericardial calcification, unless severe, is often difficult to appreciate on CMR as it causes signal loss in an already hypointense structure. Pericardial calcification is present in less than 30% of cases in developed countries, is more commonly seen in cases due to tuberculosis, and is best evaluated with CT at which time the distribution and any epicardial or myocardial extension can be noted.11
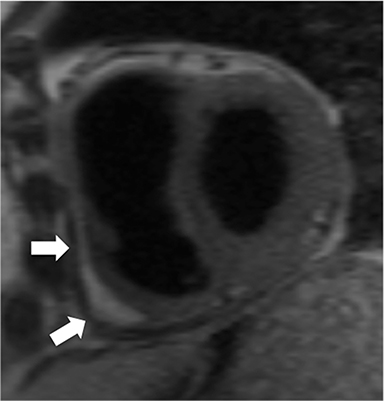
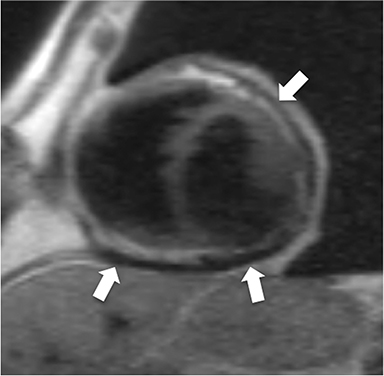
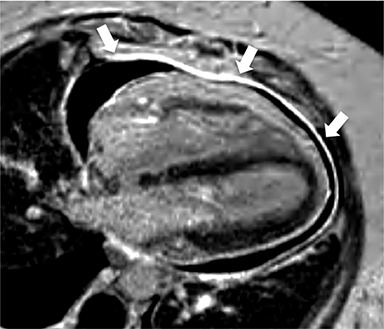
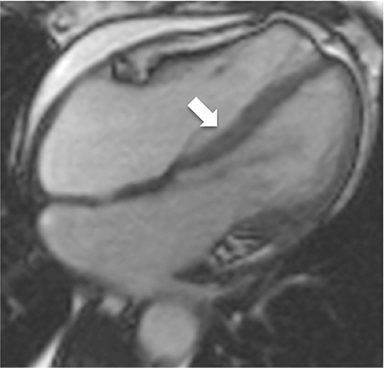
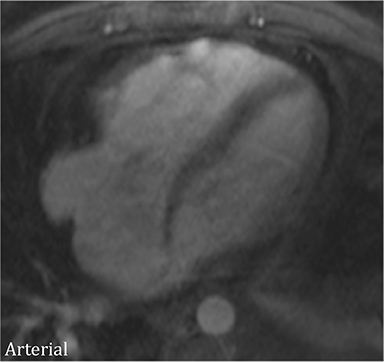
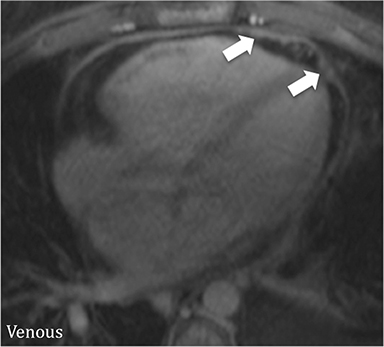
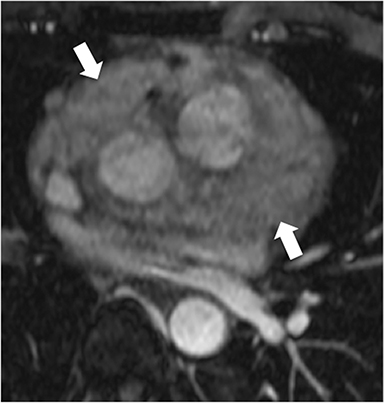
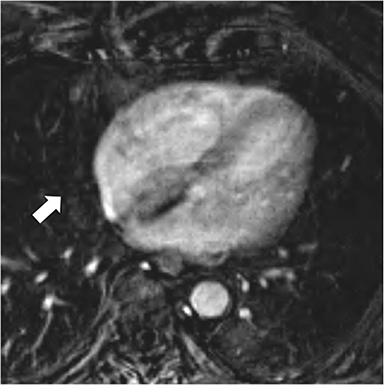
Additional morphologic findings that can be seen in CP on CMR are biatrial enlargement and narrowing and elongation of the ventricles (Figure 4). Distention of the inferior vena cava and hepatic veins is usually present and pleural effusions or ascites may be present. Myocardial-ed LGE sequences may demonstrate pericardial enhancement, a finding suggesting the presence of on-going inflammation and the potential role for immunotherapies, while simultaneously excluding many infiltrative cardiomyopathies. 12
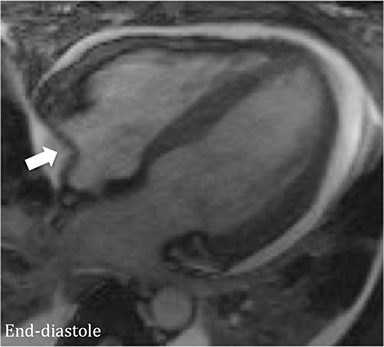
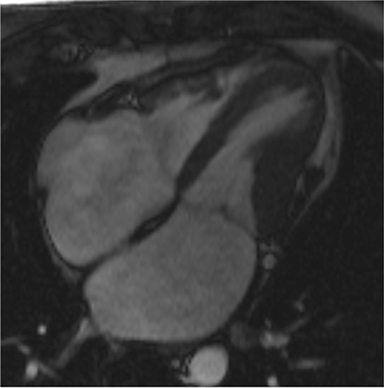
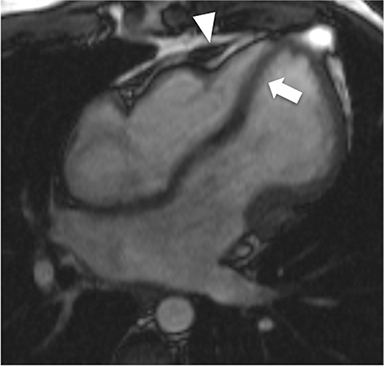
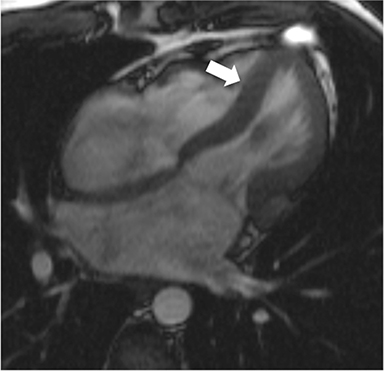
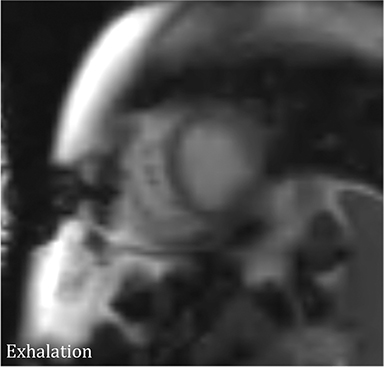
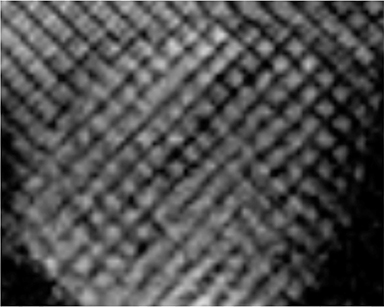
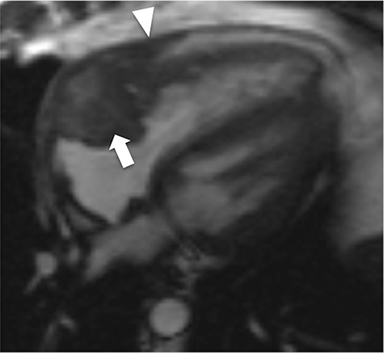
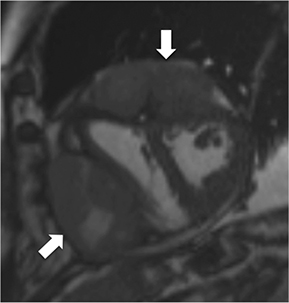
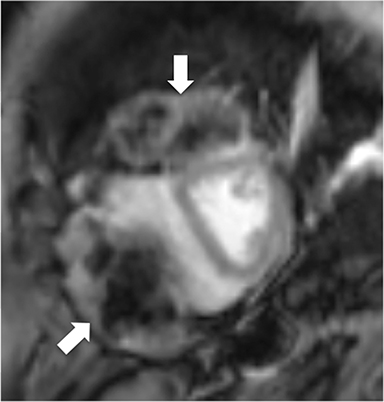
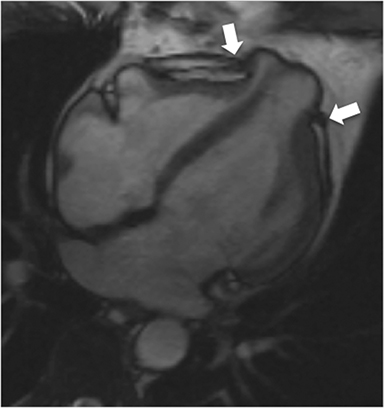
CMR’s advantage over CT comes from its ability to also provide physiologic evidence supportive of CP. Cine SSFP sequences allow for demonstration of impaired diastolic relaxation and increased interventricular dependence. While normally the pericardium exerts a mild containing force that somewhat prevents over distention of the cardiac chambers, in CP this extrinsic pressure is increased and most commonly transmitted uniformly to all cardiac chambers equalizing chamber pressures. Thus, any increase in volume to one ventricle must necessarily decrease the volume of the other ventricle. This increased ventricular interdependence is manifest as septal bounce on cine SSFP sequences where the interventricular septum variably bows from right to left during the cardiac cycle as pressures in one ventricle transiently exceed the other (Figure 5). 13 In normal circumstances, left ventricular pressures always exceed those on the right, and the septum is bowed rightward. Ventricular interdependence is best demonstrated with real time SSFP imaging in the short axis plane while asking the patient to take slow deep inhalations and exhalations (Figure 6). As there is increased systemic venous return to the right heart during inhalation, there is accentuated inhalational flattening or leftward deviation of the interventricular septum, a finding which in the appropriate clinical context has been reported to have a near 100% specificity and positive predictive value.14,15 Cine MR tag sequences in axial or four-chamber long axis orientation with tag lines perpendicular to the pericardium will demonstrate bending or persistent continuity of tag lines in patients with pericardial adhesions or CP (Figure 7). Normally, tag lines become discontinuous through the cardiac cycle as the two pericardial layers freely slide past one another.
The treatment of choice for CP is pericardial stripping. CMR can play an important role in preoperative planning by determining the presence and extent of pericardial thickening, the presence of pericardial enhancement indicating the possibility of active inflammation, and long-standing myocardial effects such as myocardial atrophy (Figure 8). 16
An uncommon but increasingly recognized entity is effusive-constrictive pericarditis (ECP). In this disease, there are overlapping features of cardiac tamponade and constriction, the latter chiefly due to inflammation and fibrosis of the visceral pericardium. The disease has been defined by the lack of normalization of right atrial pressures after pericardiocentesis.17 Thus, the patient may have symptoms in keeping with tamponade only to have constrictive effects become more prominent after pericardial fluid removal (Figure 9). While many patients may be treated with steroids, most require pericardiectomy which is more difficult given the visceral predominate disease.
Pericardial neoplasms
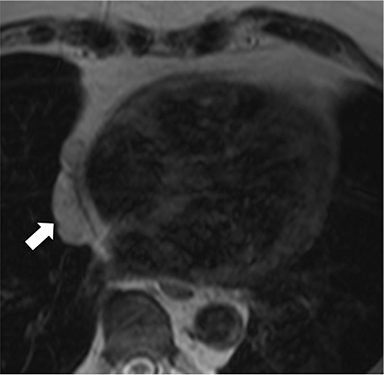
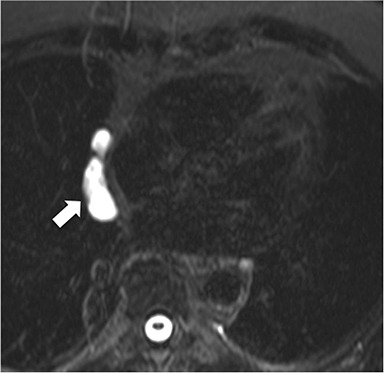
In the adult, the majority of pericardial neoplasms are malignant with the vast majority owing to metastatic disease. In autopsy series of patients dying from cancer, 10-20% have pericardial metastases.15 Metastatic involvement can occur by direct spread, hematogenous or lymphatic dissemination with lung, breast, lymphoma and melanoma among the most common culprits (Figure 10).18 Angiosarcomas arising from the right atrium have a propensity to quickly and dramatically invade the pericardial space (Figure 11). Primary pericardial neoplasms are very rare and in the adult are commonly malignant. Malignant mesothelioma is reported to be the most common primary pericardial malignancy with sarcomas and malignant teratomas among other very rare tumors (Figure 12).5,19 Benign lesions such as hemangiomas, fibromas, and lipomas are also very rare. Occasionally, prominent epicardial fat or epicardial hematoma is mistaken at echocardiography for a pericardial mass.
Pericardial neoplasms very commonly manifest with large, often hemorrhagic, pericardial effusions where the fluid component is much greater than the soft tissue component. Pericardial fluid may be variable in signal intensity depending on the degree and presence of hemorrhage. At CMR, pericardial masses are often intermediate in signal intensity on T1-weighted sequences and hyperintense on T2-weighted sequences unless extensively hemorrhagic. While not a steadfast rule, most pericardial neoplasms result in soft tissue nodularity or discontinuous pericardial thickening, frequently with soft tissue nodules appearing to extend into the epicardial fat, while infectious and inflammatory conditions more typically cause smooth, continuous pericardial enhancement. Due to the relatively avascular nature of the pericardium, neoplastic involvement may be more apparent, especially in the setting of a significant pericardial effusion, on more delayed-phase, postcontrast images.20 With the exception of fat containing lesions such as lipomas, liposarcomas or teratomas, the majority of pericardial neoplasms cannot reliably be differentiated by tissue characterization on CMR; rather the benefit of CMR lies in accurate localization of lesions and identification of invasion of the pericardium and/or myocardium. Whenever pericardial masses are encountered, the amount of pericardial effusion and any evidence of tamponade effect should be reported. In most cases, diagnosis is established by simultaneous pericardiocentesis and pericardial window to protect against future development of tamponade.
Congenital anomalies
Pericardial cyst is the most common benign pericardial mass and arises during development as an encapsulated cyst separate and isolated from the pericardial space. They occur most commonly in the right greater than left cardiophrenic angles and rarely in the anterior mediastinum.21 Since the vast majority are asymptomatic, they are most commonly discovered incidentally on studies performed for other reasons or mistaken for mediastinal or pulmonary masses on chest radiography.
CMR readily demonstrates the cystic nature of these lesions, which have imperceptible walls, T1 hypointensity, T2 hyperintensity and no enhancement on post-contrast imaging (Figure 13). Sometimes due to more proteinaceous composition of the cyst fluid the density can mimic soft tissue attenuation on CT and raise the T1 signal on CMR; however, STIR and more heavily weighted T2 sequences will demonstrate the lesion to be cystic with high signal intensity. Rarely, pericardial cysts may become superinfected which can cause heterogeneous T1 and T2 signal characteristics, and occasionally they can exert mass effect on adjacent cardiac chambers. While pericardial cysts can grow slowly over time, rapid temporal or positional change in size should raise the question of pericardial diverticulum or loculated pericardial effusion in the setting of adhesions. 1,20
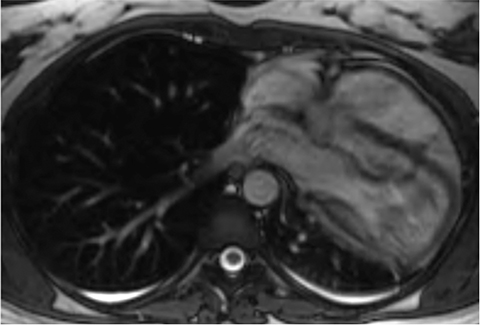
Partial or complete absence of the pericardium is another rare congenital anomaly that is often incidentally detected. Partial absence is more common on the left than right and depending on the size of the defect can predispose to herniation of cardiac chambers impairing their function and/or causing ischemic necrosis or result in compression of the epicardial coronary arteries.15 Complete absence is more rarely encountered and is considered benign.
On CMR, the pericardium itself along the left heart is often difficult to visualize given the paucity of epicardial and pericardial fat in these regions. Therefore, secondary evidence of partial absence of the left pericardium is key in confirming the diagnosis such as excessive leftward deviation of the cardiac apex and lung interposed between the heart and diaphragm as well as lung extending between the aorta and pulmonary artery in the aorticopulmonary window, a finding considered pathognomonic for this condition (Figure 14).22 Cine SSFP sequences can show focal restriction of chamber motion at the site of the defect or reveal excessive motion of the left ventricular apex which is normally relatively stationary given the pericardial anchoring to the diaphragm. In the setting of chamber or coronary compromise, surgical correction may be undertaken.
Conclusion
Diseases and disorders of the pericardium can have significant clinical implications. While echocardiography is the first line approach in evaluation, CMR can play an important adjunct role when there is clinical uncertainty or a need for more comprehensive anatomic and physiologic evaluation.
References
- Bogaert J, Francone M. Pericardial disease: value of CT and MR imaging. Radiology. 2013;267:340-356.
- Kovanlikaya A, Burke LP, Nelson MD, Wood J. Characterizing chronic pericarditis using steady-state free-precession cine MR imaging. AJR Am J Roentgenol. 2002 ;179:475-476.
- Klein AL, Abbara S, Agler DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2013 ;26:965-1012.
- Dudzinski DM, Mak GS, Hung JW. Pericardial diseases. Curr Probl Cardiol. 2012;37:75-118.
- Kim JS, Kim HH, Yoon Y. Imaging of pericardial diseases. Clin Radiol. 2007 ;62:626-631.
- Alter P, Figiel JH, Rupp TP, et al. MR, CT, and PET imaging in pericardial disease. Heart Fail Rev. 2013 ;18:289-306.
- Young PM, Glockner JF, Williamson EE, et al. MR imaging findings in 76 consecutive surgically proven cases of pericardial disease with CT and pathologic correlation. Int J Cardiovasc Imaging. 2012 ;28:1099-1109.
- Misselt AJ, Harris SR, Glockner J, et al. MR imaging of the pericardium. Magn Reson Imaging Clin N Am. 2008 ;16:185-199.
- Restrepo CS, Lemos DF, Lemos JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27:1595-1610.
- Talreja DR, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852-1857.
- Oh KY, Shimizu M, Edwards WD, et al. Surgical pathology of the parietal pericardium: a study of 344 cases (1993-1999). Cardiovasc Pathol. 2001 Jul-;10:157-168.
- Feng D, Glockner J, Kim K, et al. Cardiac magnetic resonance imaging pericardial late gadolinium enhancement and elevated inflammatory markers can predict the revers ibility of constrictive pericarditis after anti-inflammatory medical therapy: a pilot study. Circulation. 2011 25;124:1830-1837.
- Giorgi B, Mollet NR, Dymarkowski S, et al. Clinically suspected constrictive pericarditis: MR imaging assessment of ventricular septal motion and configuration in patients and healthy subjects. Radiology. 2003 ;228:417-424.
- Francone M, Dymarkowski S, Kalantzi M, et al. Assessment of ventricular coupling with real-time cine MRI and its value to differentiate constrictive pericarditis from restrictive cardiomyopathy. Eur Radiol. 2006 ;16:944-951.
- Peebles CR, Shambrook JS, Harden SP. Pericardial disease—anatomy and function. Br J Radiol. 2011 ;84 Spec No 3:S324-37.
- Rienmuller R, Groll R, Lipton MJ. CT and MR imaging of pericardial disease. Radiol Clin North Am. 2004 ;42:587-601.
- Syed FF, Ntsekhe M, Mayosi BM, Oh JK. Effusive-constrictive pericarditis. Heart Fail Rev. 2013 ;18:277-287.
- Chiles C, Woodard PK, Gutierrez FR, Link KM. Metastatic involvement of the heart and pericardium: CT and MR imaging. Radiographics. 2001 ;21439-449.
- Grebenc ML, Rosado de Christenson ML, Burke AP, et al. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. 2000;20:1073-1103.
- Lopez Costa I, Bhalla S. Computed tomography and magnetic resonance imaging of the pericardium. Semin Roentgenol. 2008;43:234-245.
- Yared K, Baggish AL, Picard MH, et al. Multimodality imaging of pericardial diseases. JACC Cardiovasc Imaging. 2010 ;3:650-660.
- Soulen RL. Magnetic resonance imaging of great vessel, myocardial, and pericardial disease. Circulation. 1991 ;84(3 Suppl):I311-1321.