MDCT and 3D imaging of the small bowel and mesentery








The utilization of cross-sectional imaging in the evaluation of the small bowel has been growing in recent years, with a significant shift away from fluoroscopic imaging modalities using positive enteric contrast, and toward cross-sectional imaging, primarily computed tomography (CT).
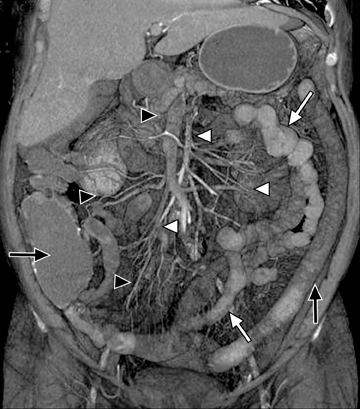
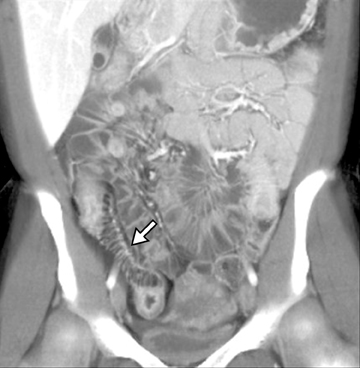
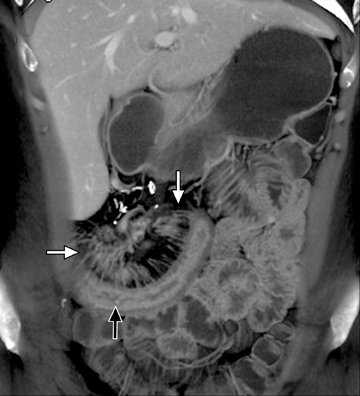
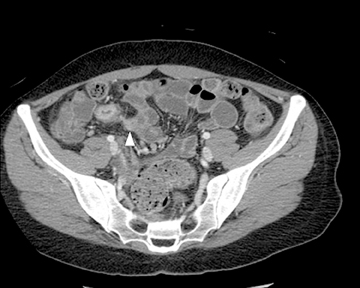
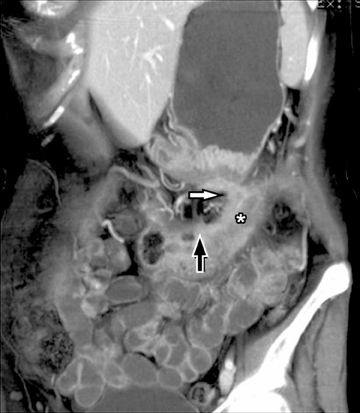
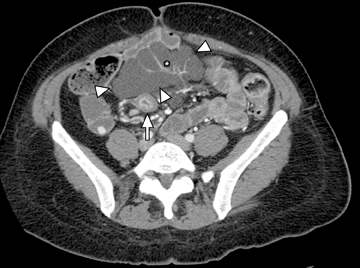
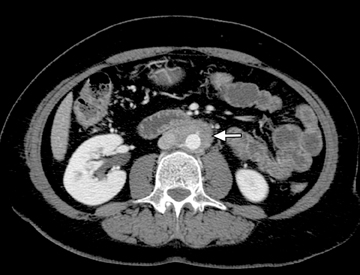
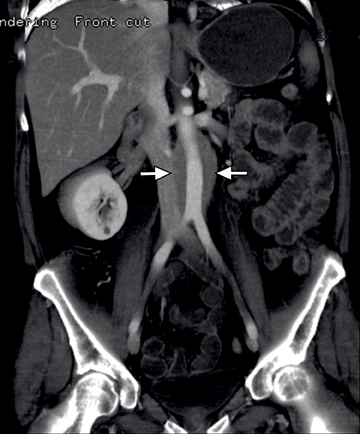
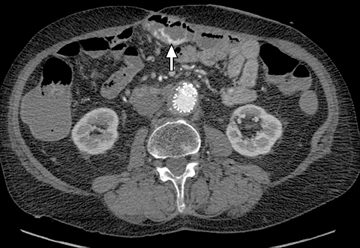
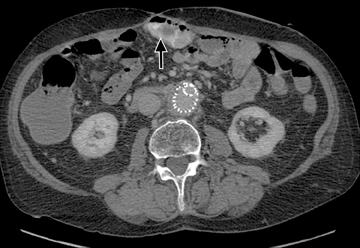
This change is attributed to several technological advances in CT imaging, including the introduction of multidetector technology that began with 4 slices and moved to, more recently, clinically available scanners offering up to 320-detector rows. Multidetector CT (MDCT) allows for the acquisition of multiple image slices with every gantry rotation, improving temporal and spatial resolution of the examination and increasing the area of coverage during a short interval for homogeneous acquisition in a single-phase of enhancement. The significantly shorter scanning time improves visualization and assessment of the small bowel by decreasing motion artifacts and allowing rapid scan acquisition at multiple phases of enhancement. Decreasing slice thickness, on the other hand, improves spatial resolution, leading to acquisition of nearly isotropic voxels. This, in turn, improves assessment of small structures, such as terminal mesenteric vessels, and allows for optimal multiplanar and 3-dimensional-volumetric reconstruction of the bowel and mesenteric vessels (Figure 1).
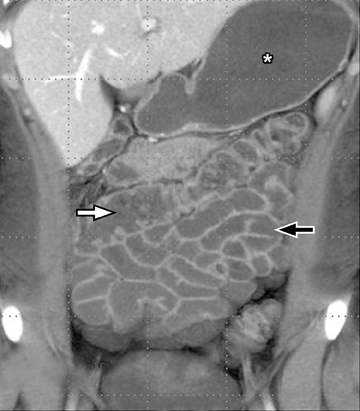
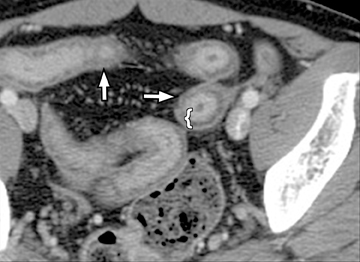
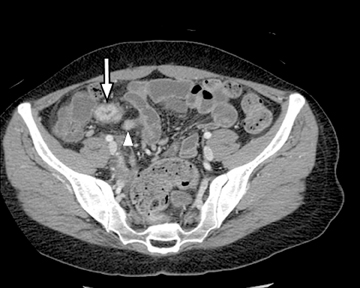
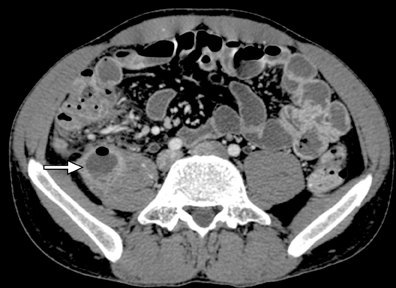
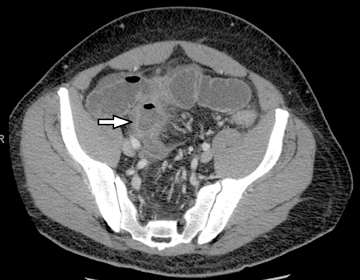
A second, and probably equally, important advance that has improved assessment of the small bowel is negative (neutral or low Hounsfield units (HU)) enteric contrast. The low, near-water density of the new oral contrast permits assessment of the mucosal density or enhancement pattern, in the background of the low attenuation within the lumen, which may be affected by multiple pathologies such as inflammation or masses (Figure 2). Since the CT examination is optimized in technique and use of oral contrast to evaluate the enteric system, it is usually referred to as CT enterography (CTE).
This article reviews CTE techniques for optimizing small bowel and mesentery evaluation, including mesenteric vessels, and reviews the appearances of common pathologies that can be evaluated more effectively with current MDCT with an emphasis on the evaluation of patients with inflammatory bowel disease.
MDCT scan parameters
Multidetector CT scanners allow acquisition of several slices, depending on detector configuration, during a single gantry rotation. These range from 4- to 320-multirow detectors, depending on scanner type and manufacturer. Depending on the detector configuration, extremely thin, submillimeter slices can be acquired. The imaging slices can then be reconstructed in any thickness, commonly between 2 and 3 mm, preserving the quality of the examination while decreasing the number of images for easier review. The thinner slices are used for problem solving and for generating 3-dimensional and multiplanar images.1 The nearly isotropic voxel acquisition offers an additional advantage in bowel imaging, allowing for improved spatial resolution, better visualization of the mucosa, and appreciation of the different bowel wall layers, especially in disease states.
Timing of scan acquisition
After intravenous (IV) contrast administration, the small bowel can be imaged in 4 different phases of enhancement: arterial, enteric, venous, and delayed. The selection of scan timing and number of acquisition phases to optimize evaluation depends primarily on clinical presentation of the suspected pathology. In evaluating patients with Crohn’s disease, a single enteric or venous phase scan is considered adequate for evaluation of bowel-wall abnormalities and enhancement patterns, and to assess for extra-intestinal complications.2
For imaging small bowel tumors and assessing vascular abnormalities a biphasic evaluation in the arterial and enteric or venous phases at minimum is essential to detect arterially enhancing lesions, to assess the patency of vessels, and to evaluate the solid organs for focal abnormalities, such as metastases. More delayed phases may also be obtained in the investigation of occult gastrointestinal (GI) bleeding for improved sensitivity and characterization of small vascular malformations or minute amounts of bleeding.3,4 Bolus-tracking or bolus-triggered methods for the acquisition time can be optimized at the intended phase to get the arterial phase, immediately followed by the enteric phase (20 to 25 sec) or delayed phase (70 to 75 sec) after the beginning of the arterial phase acquisition.5 Our CTE protocol is performed after administration of 125 cc of Isovue-370 (Bracco Diagnostics Inc, Princeton, NJ) at an injection rate of 4 cc/sec followed by a 50 cc saline flush injected at a rate of 4 cc/sec. The scan is then acquired 65 sec from the start of the injection. For arterial phase imaging in dual-phase scanning, the scan is triggered by a bolus-automated technique starting 3 sec after a threshold density of 150 HU is reached in the abdominal aorta.
Oral contrast
The routine use of positive enteric contrast (barium- or iodine-based), with its high attenuation, can mask visualization of the mucosal enhancement pattern and also may limit scan sensitivity to hyper-attenuating intraluminal or bowel wall lesions. It also may interfere with volumetric reconstruction for assessing enhancing structures, especially vessels. The introduction of negative (low HU or neutral) enteric contrast accomplished 2 important tasks. The first was to optimize bowel distention, which helps eliminate apparent bowel wall thickening, and the second was to improve assessment of mucosal hyperattenuation. Both of these can create false-positive results in interpretation (Figure 3).
Historically, water has been used to distend the bowel; however, the use of water is limited in the distention of distal small bowel, as most of it will be absorbed by the time it reaches the ileum. Newer agents include an array of low-attenuation materials, such as Mucofalk, mannitol, methylcellulose solution, polyethylene glycol electrolyte solution, or low-concentration barium.6-8 We use VoLumen (Bracco Diagnostics Inc, Princeton, NJ) in our department as a neutral oral contrast agent that consists of barium sulphate suspension (0.1% w/v, 0.1% w/w). The oral contrast is taken over the course of 1 hr before the examination with a total approximate amount given of about 1800 ml split into 450 ml administered at 1 hr, 450 ml at 40 min, 225 ml at 20 min and another 225 ml immediately before the scan. Some patients, especially those with obstructive symptoms, may not be able to consume the total dose of contrast; water can be substituted for the last dose(s), since absorption is not an issue in distending the stomach or duodenum.
Small bowel and mesentery evaluation
The small bowel can be affected by a wide variety of disorders, including inflammation, infection, ischemia, and malignancy. CTE is used most commonly in the evaluation of patients with suspected inflammatory diseases.9-12 Inflammatory bowel diseases (IBD) encompass Crohn’s disease (CD), intermediate colitis, and ulcerative colitis (UC). Crohn’s disease is a transmural inflammatory disease that involves the small bowel, most commonly the terminal ileum, with or without upper gastro-duodenal or colonic involvement. The terminal ileum can, however, sometimes be involved with intermediate colitis and ulcerative colitis in the form of back-wash ileitis, which is usually contiguous with large bowel disease. Characteristically, Crohn’s disease demonstrates multiple skip areas of involvement with intervening unaffected segments in the more proximal small bowel, including the duodenum and stomach as well as the colon. This is in contrast to the two other forms of IBD that usually affect the colon in a contiguous fashion, starting distally at the level of the colon.
The clinical workup of patients with Crohn’s disease usually involves optical upper and lower endoscopy; however, most of the small bowel between the duodenum and terminal ileum is inaccessible with these techniques, limiting evaluation and missing important areas of potential involvement. Another endoscopic “blind spot” lies in evaluating extra-intestinal complications of IBD. A more recently available clinical modality for evaluating the small bowel is capsule endoscopy, which can image the entire small bowel mucosa. However, this is also limited in visualizing extra-intestinal disease and can potentially be complicated by retention of the capsule in a localized high-grade stricture at sites of disease. This can lead to obstruction that may require surgical intervention (Figure 4).
Historically, patients with Crohn’s disease have had radiologic work-ups with small bowel follow-up examinations (SBFT) that could provide details of mucosal edema and assess the presence of strictures and fistulas/sinus tract. However, these exams were limited in their ability to evaluate the extra-enteric complications of IBD, especially abscess formation.
They also typically require up to several hours to perform, especially in patients with obstruction, in addition to the difficulty in separating overlapping bowels that may limit evaluation. CTE evaluation, on the other hand, provides several advantages over SBFT, including: more rapid acquisition, requiring only a few seconds to obtain the diagnostic scan (after the oral prep); assessment of the majority of the GI system in the same setting (stomach, duodenum, small and large bowel); assessment of the location, extent, and number of diseased segments; detecting the presence or absence of bowel obstruction; more accurate assessment of disease activity by assessing the presence or absence of intrinsic mucosal and transmural inflammatory bowel changes; and detecting extra-intestinal complications.
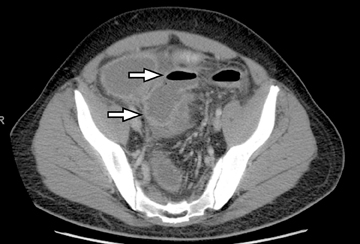
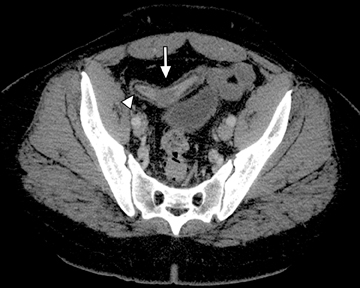
The common signs of active Crohn’s disease include: increased mucosal enhancement, bowel wall thickening, mural stratification, stranding of the surrounding fat and engorgement of the mesenteric vessels supplying the diseased bowel segment (Figures 5-7).6 However, CTE has poor sensitivity for assessing fibrotic strictures due to the inability of CT to characterize fibrosis or collagen deposition, and presence of fibrosis is usually presumed if the typical inflammatory changes are absent. CTE can assess for the presence of penetrating disease, detecting sinus or fistula tracts extending from the diseased segment that are commonly characterized by bowel tethering and visualization of linear tracts that may communicate with adjacent structures, such as bowel or extraperitoneal spaces (most commonly entero-enteric, entero-colic, enterocutaneous) (Figures 8 and 9).
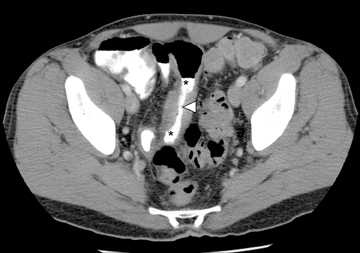
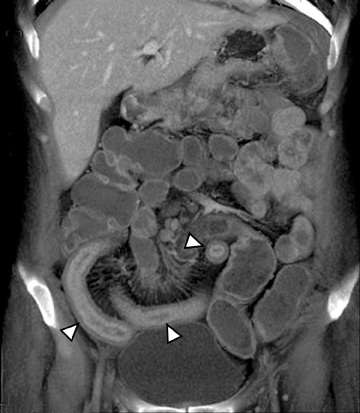
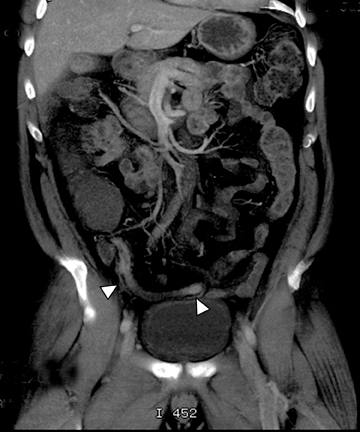
Another sign of penetrating disease is abscess formation that is usually contiguous to the diseased segment and can be seen in the peritoneal cavity or retroperitoneal space (Figure 10). One important potential pitfall in the use of neutral oral contrast is the detection of small mesenteric fluid collections that appear similar in density to adjacent bowel loops (Figure 11). This problem can be overcome by carefully following the bowel lumen and separating the intraluminal fluid from the extraluminal fluid, as well as by performing multiplanar projection for better display of the bowels in the coronal or sagittal plane. When patients with Crohn’s disease present with signs of acute flare or obstruction, the main advantage of CTE is to assess the underlying cause, which would determine clinical management.13
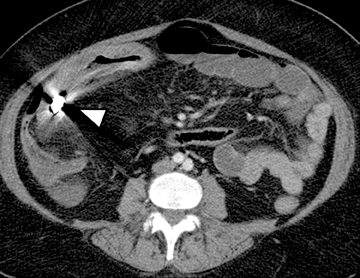
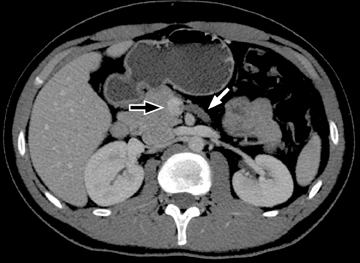
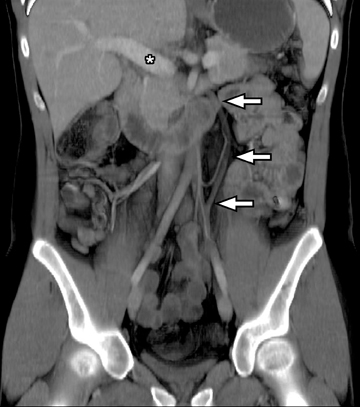
After detecting small bowel strictures it becomes imperative to differentiate inflammatory strictures showing signs of active inflammation that are managed mostly medically from fibrostenosing strictures that are predominantly fibrotic and that may require surgical intervention (Figure12). Frequently, CTE is also used in the postoperative evaluation of Crohn’s disease patients with symptoms suggesting recurrent disease; most frequently seen at or immediately proximal to the anastomotic site (Figure13). Not uncommonly, very low attenuation can be noted within the bowel wall in patients with a history of longstanding IBD (both CD and UC) due to fat deposition within the submucosal layer of the bowel (Figure 14).14 The presence of fat is not necessarily indicative of quiescent disease; fat may be present with signs of active inflammation. An additional potential CTE indication is its use as a baseline examination before starting a new treatment—especially one with biologic agents, which commonly cause immunosuppression—to exclude abscess formation, or as a follow-up tool to assess treatment response.15 CTE can also help to evaluate vascular patency in patients with IBD or other suspected infectious or inflammatory bowel conditions (Figure 15).
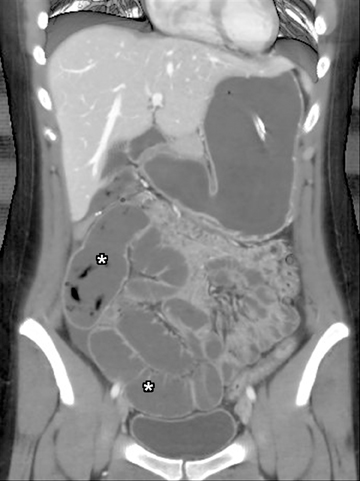
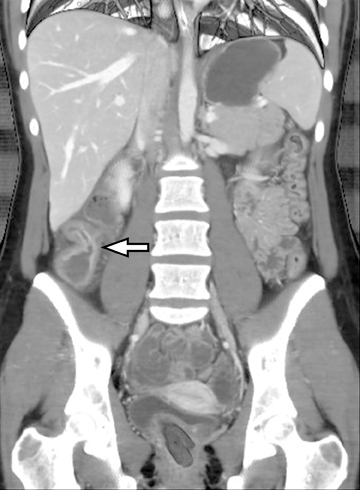
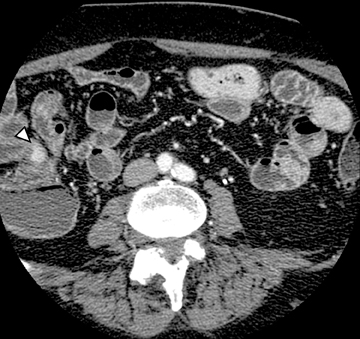
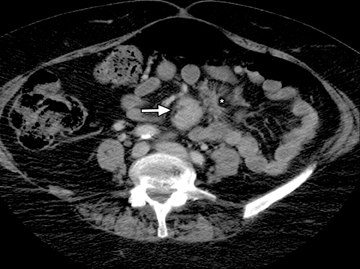
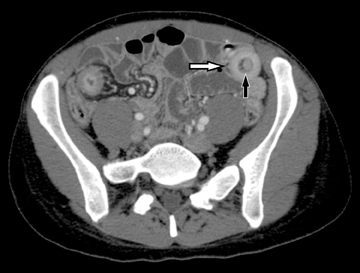
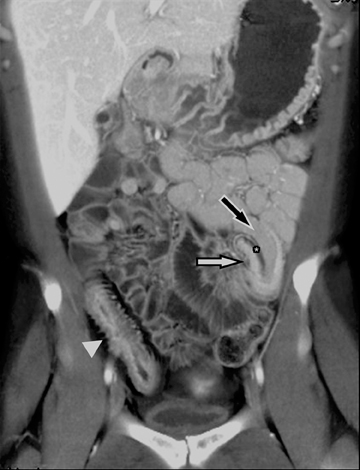
An additional potential clinical indication for CTE is the assessment of small bowel tumors, mesenteric vessels or vascular lesions. These examinations can be optimized by using biphasic arterial and enteric or venous phase scans that improve both detection and characterization of suspected abnormalities. Commonly encountered small bowel malignancies include primary lesions, such as carcinoid tumors (Figure16), primary adenocarcinoma, and GI tumors, as well as metastatic lesions from a variety of primary tumors that include other GI malignancies, melanoma, and breast and lung carcinomas. A variety of incidental findings can be detected on CTE that may be related to the bowels or within the abdomen and pelvis (Figures17-19). Multiphase CT can also be used to evaluate for active GI bleeding, which is diagnosed when high-attenuation, extravasated contrast material is seen accumulating, and later diffusing, within the bowel lumen (Figure 20). Common causes of upper GI bleeding include erosions or ulcers, variceal bleeding, Mallory-Weiss tears, vascular lesions, and neoplasms.16
Conclusion
Recent advances in CT, most importantly multidetector CT technology and neutral oral contrast, are leading to a shift toward cross-sectional imaging and away from fluoroscopic examination in evaluation of patients with suspected small bowel or mesenteric disorders.
The most common current indication for single-phase CTE is evaluation of patients with inflammatory bowel disease, especially Crohn’s disease, in which the modality has shown several advantages over other modalities, such as endosocpy or SBFT. Careful consideration of patients for repeated imaging or multiphase scanning is essential due to concern about lifetime cumulative radiation dose.
References
- Huprich JE, Fletcher JG. CT enterography: Principles, technique and utility in Crohn’s disease. Eur J Radiol. 2009;69:393-397.
- Fletcher JG. CT enterography technique: Theme and variations. Abdom Imaging. 2009;34:283-288.
- Huprich JE, Fletcher JG, Alexander JA, et al. Obscure gastrointestinal bleeding: Evaluation with 64-section multiphase CT enterography—initial experience. Radiology. 2008;246:562-571.
- Scheffel H, Pfammatter T, Wildi S, et al. Acute gastrointestinal bleeding: Detection of source and etiology with multidetector-row CT. Eur Radiol. 2007;17:1555-1565.
- Graça BM, Freire PA, Brito JB et al. Gastroenterologic and radiologic approach to obscure gastrointestinal bleeding: How, why, and when? Radiographics. 2010;30:235-252.
- Paulsen SR, Huprich JE, Fletcher JG, et al. CT enterography as a diagnostic tool in evaluating small bowel disorders: Review of clinical experience with over 700 cases. Radiographics. 2006;26:641-57.
- Reittner P, Goritschnig T, Petritsch W, et al. Multiplanar spiral CT enterography in patients with Crohn’s disease using a negative oral contrast material: Initial results of a noninvasive imaging approach. Eur Radiol. 2002;12:2253-2257.
- Zhang LH, Zhang SZ, Hu HJ, et al. Multi-detector CT enterography with iso-osmotic mannitol as oral contrast for detecting small bowel disease. World J Gastroenterol. 2005;11: 2324-2329.
- Solem CA, Loftus EV Jr., Fletcher JG, et. al. Small-bowel imaging in Crohn’s disease: A prospective, blinded, 4-way comparison trial. Gastrointest Endosc. 2008;68:255-266.
- Vogel J, da Luz Moreira A, Baker M, et al. CT enterography for Crohn’s disease: Accurate preoperative diagnostic imaging. Dis Colon Rectum. 2007;50:1761-1769.
- Hara AK, Swartz PG. CT enterography of Crohn’s disease. Abdom Imaging. 2009;34:289-295.
- Lee SS, Kim AY, Yang SK, et al. Crohn disease of the small bowel: Comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751-761.
- Higgins PD, Caoili E, Zimmermann M, et al. Computed tomographic enterography adds information to clinical management in small bowel Crohn’s disease. Inflamm Bowel Dis. 2007;13:262-268.
- Wittenberg J, Harisinghani MG, Jhaveri K, et al. Algorithmic approach to CT diagnosis of the abnormal bowel wall. Radiographics. 2002;22:1093-1107.
- Hara AK, Alam S, Heigh RI, et al. Using CT enterography to monitor Crohn’s disease activity: A preliminary study. Am J Roentgenol. 2008;190:1512-1516.
- Laing CJ, Tobias T, Rosenblum DI, et al. Acute gastrointestinal bleeding: Emerging role of multidetector CT angiography and review of current imaging techniques. Radiographics. 2007;27:1055-1070.
