Improving screening mammography: Perspective of a community radiologist
Images
The United States Preventative Services Task Force (USPSTF) recommendations of 2009 have impacted mammographic screening to good and ill effect. First and foremost, these recommendations have aggravated an already smoldering discussion on whether and when to screen, created confusion in the lay public, and resulted in a drop in mammographic screening procedures.
To review, the USPSTF published new recommendations that differed sharply from those of the American Cancer Society (ACS), the American College of Radiology (ACR) and the American College of Obstetricians and Gynecologists (ACOG). For women aged 40 to 49 years, the USPSTF “recommends against routine screening mammography.” Instead, the task force issued a “C” recommendation, which states, “Clinicians may provide this service to selected patients depending on individual circumstances. However, for most individuals without signs or symptoms, there is likely to be only a small benefit from this service.” The USPSTF also recommended a switch from annual to biennial screening mammography in women aged 50 to 74 years (a “B” recommendation). Regarding women aged 75 years, the task force issued an “I” statement, thus concluding that current evidence is insufficient to assess the additional benefits and harms of screening mammography.
The controversial recommendations were widely publicized by the media, creating confusion for physicians and patients alike. Until 2009, screening mammography demonstrated increased utilization at a slow, steadily increasing rate of approximately 1% between 2002 and 2009, ranging from 271 screenings per 1000 women in 2002 to approximately 322 screenings per 1000 women in 2009, the year the guidelines were issued. The following year, 2010, the screening rate dropped to 309 per 1000 women; a 4.3% decrement in utilization. This equivalent loss of four years of growth in a single year is by any standard remarkable. Assuming a lifetime cancer rate of 12% in the general population, the drop potentially translates into one to two undetected cancers at the new utilization rate. An analysis using the Cancer Intervention and Surveillance Modeling Network indicates that the USPSTF recommended screening regimen misses 20 to 25 more cancers per 1000 women screened than the ACS-recommended screening regimen and costs approximately 6,500 more women’s lives per year.3 Since then, discussion in professional circles has focused on topics of overdiagnosis, actual mortality reduction, reasons for mortality reduction and radiation exposure risk vs. benefit of screening for various age groups.
Overdiagnosis is estimated as the difference between disease detected with screening that would not have been diagnosed in the host’s lifetime if screening had not taken place. Critics of screening indicate that detection of disease can lead to approximately 41% to 46% more overdiagnoses in women who are screened beginning by age 40 to 50.4 Advocates argue that the most commonly accepted definition of overdiagnosis is not a pathologic one, but rather an epidemiologic one. They argue that critics ignore the pathologic features that can differentiate a progressive cancer from a nonprogressive cancer, which is critical to screening, and many of the detected cancers are at a stage in which there is sufficient lead time for a cure. Screening must advance the time of diagnosis within the clinically occult phase when treatment has the greatest potential for success; ie, there must be sufficient lead time and therefore, there is a potential for apparent overdiagnosis. Advocates argue that true overdiagnosis is estimated at <10%.5
Arguments over the reasons for breast cancer mortality reduction include increased screening mammography, increased awareness of breast cancer and improved therapies. Which factor has the greatest influence on the 30% mortality decrease is still being debated.4-7 The estimated reduction is based on several random controlled population studies.6 It is generally agreed that between 1997 and 2007, U.S. mortality from breast cancer decreased 31%, principally due to mammographic screening and improved therapies. Furthermore, it is argued that the detection of smaller lesions allows for more effective therapy and greater chance of patient survival.5, 7 Awareness of increasing radiation exposure in the medical community has created concern for excess unnecessary exposure in mammography. All of these issues beg the question of the shortcomings of mammography.
Although the USPSTF guidelines have had a negative imaging impact and possibly negative impact on cancer detection, they have brought to the forefront some deficiencies of mammography and further ignited the discussion for increased sensitivity and improved specificity among breast imagers. I believe breast imagers will rise to the USPSTF challenge to improve screening, diagnostic interpretations and outcomes, which brings us to the focus of this article.
Screening mammography has been the workhorse of breast cancer detection for over 40 years, yet its sensitivity is variable, in large part based on breast density. Dense tissue is a common, present in more than half of women younger than 50 years and nearly a third of women older than 50 years. Therefore, breast cancer sensitivity for detection can range from 30% to 48% in women with dense breasts, to as high as 80% to 98% in women with fatty breasts. It is in women with dense breasts that radiologists are the most challenged to find or exclude malignancy. These are the patients that account for the most recalls, higher rates of recommended biopsies, higher rates of false positive biopsies and greatest radiation exposure. Endeavoring to correct these deficiencies has been and continues to be the thrust of the breast imaging profession.
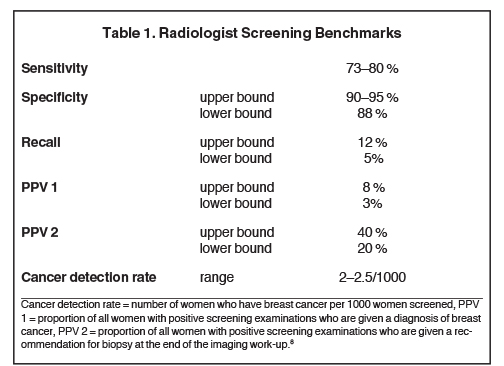
The ACR remains on the forefront of not only advocating screening as the best tool, but of recognizing the need for improvement. This can occur in two major areas: technological improvements and personnel training. By far, the majority of mammographic screening occurs at the community level, where it is performed by community radiologists such as myself. This is where the greatest impact on women’s breast health occurs. Obviously, as community breast imagers, it is our mission to maximize accuracy. This requires constant practice to develop a comfortable and capable experience, and continuing education to augment our knowledge which allows us to keep up to date on the best imaging algorithms and to maintain adequate benchmarks for screening and diagnostic mammography. Most, if not all, breast imagers are or should be aware of the performance benchmarks for a typical screening population which have been established over several years of data collection at regional registries and academic centers.8 A study published in 2015 updated and further broadened acceptable performance parameters using combined criteria that considered dependence of various performance measures.9
In today’s healthcare market, where patients have become accustomed to participating in their care by questioning physicians, their medical care delivery and record of care delivery, so too patients and their referring physicians should ask what the performance benchmarks are for the breast imaging radiologist. The criteria to identify thresholds for minimally acceptable physician performance in interpreting screening mammography are presented in Table 1. One way to improve mammography and address some of the issues raised by the USPSTF, is to identify areas that radiologists can change and improve upon. In a recent study, in which I participated, the agreement of mammographic interpretations by community radiologists with consensus interpretations of an expert radiology panel was undertaken to identify approaches that may improve mammographic performance.10
The multi-center trial identified mammographic asymmetries and architectural distortions as areas of decreased agreement when comparing community and expert radiologists. Thus, these two findings are recognized as areas in which training should be focused to improve screening outcomes and reduce recalls. Most important is the improvement of outcomes allowing better discrimination between those findings that warrant additional workup and those that do not. Equally important is the reduction of recall rate, hence, a potential positive impact by decreasing false positive biopsies, morbidity and reduction of unnecessary radiation exposure. The most recent technological advance available to breast imagers is tomosynthesis with computed tomographic (CT) mammography. Breast tomosynthesis has been shown to reduce the number of recalls for asymmetries.11 An ongoing prospective population screening study using mammography plus tomosynthesis has demonstrated a 15% reduction in recall rates; ie, false positives, and a 27% increase in the cancer detection rate.12
Radiation reduction
Radiation exposure is of increasing concern with the explosion of medical imaging which, fueled in part by the development of indispensable, readily available imaging resources, has exhibited a six-fold per capita dose increase of ionizing radiation between the 1980s and the present. The average doses delivered in the Digital Mammography Imaging Screening Trial, or DMIST, fell from 4.7 mGy for screen-film mammography to 3.7 mGy for digital mammography for a standard examination with two views per breast.13 Most if not all imaging centers and community hospitals provide lower-dose digital services for women. The authors proceeded to show through modeling that in a cohort of 100,000 women, mammographic screening that would be conducted annually from ages 40 to 55 years and biennially until age 74 at a dose of 3.7 mGy per examination would ultimately induce 86 breast cancers. For the screening regimen given above, it is estimated that 11 deaths attributable to radiation-induced breast cancer would occur. For the same regimen over the same period of 34 years, 136 woman-years would be lost per 100,000 women in the cohort due to radiation-induced cancer, but 10,670 woman-years would be saved by earlier detection through screening. The implication for women’s health care is that the risk of radiation-induced breast cancer associated with routine mammographic screening of women 40 years of age and older and the number of deaths expected due to such cancers are extremely low, especially when compared with the expected benefits from screening. Radiation risk, while remaining a global concern, should not be a deterrent from screening in these women.
Non-radiation alternatives to mammography are available, albeit with variable sensitivities and specificities which have yet to undergo randomized controlled population studies. These studies will take decades to validate the modalities as mammography has been validated, however, in the meantime they have proven very useful in breast cancer detection. One readily available and inexpensive non-radiation modality is breast ultrasound. Recently, there has been significant publicity regarding screening of dense breasts by ultrasound because of the detection shortcomings of mammography in patients with dense breasts.14 The issue has reached political circles with the implementation of Connecticut Public Act 09-41 requiring radiologists to inform patients with heterogeneous or extremely dense breasts at mammography that they may benefit from an ultrasound examination. Since then nearly 26 states have either enacted or are in the process of enacting, forming, or advising similar legislation.
A recent “screening” study of ultrasonography performed on women with heterogeneously dense or dense breasts performed in Connecticut at Yale University demonstrated increased cancer detection.14 The overall cancer yield was 3.2 cancers per 1000 women screened, which is comparable to screening mammography alone (Table 1). Although the results are encouraging, the study raised several issues in the radiology community which are significant and need mention.15 First, the study claimed to be a screen; however, by strict definition, the study included screened and diagnostic patient populations. This resulted in skewing of the outcomes to appear more favorable than expected from a true screening population. Second, the skewed data can and likely have influenced lawmakers’ decisions to promulgate similar legislation in other states. It is felt, in many breast imaging circles, that the mandate is premature and yet to be validated by pure screen and pure diagnostic random trial studies.
Since cancer detection is the main issue in “dense” breasts, the discussion then becomes what to do. When density is mandated to be presented to women in the lay letter, it raises this issue to a level of great anxiety which, at present is not supportable. The obvious next question is what to tell women who have received the letter. The only semi-logical answer is to add another modality or other modalities in an effort to counterbalance the deleterious detection effects of “density.” The two major modalities are US and MRI, each of which bring their own not insignificant challenges, including high false-positive rates. US provides real-time imaging, but is time consuming while MRI is highly sensitive but not real time, expensive and has higher false-positive outcomes than ultrasound.
A proposed solution is not to start at the top with legislation, but to begin, in earnest, a wide educational program to clearly state the risks of dense breasts and solutions we currently have to deal with that issue, especially with detection of early breast cancer (Personal communication with Carl J. D’Orsi, MD, Director of Breast Imaging Research, Department of Radiology, University Breast Imaging Center, Emory University, Atlanta, Ga., 2014).
The need remains to perform valid comparisons of breast imaging technologies and to critically assess whether their greatest impact would be in a screening, the diagnostic setting, or both. The proposed South Carolina Bill 0422, which would have mandated language requiring reportage of the breast density was recently taken off the legislature’s agenda (Personal communication with Timothy Pierce, MD, President, South Carolina Medical Association, 2014).
Regardless of the argument facing breast imagers, the horse is out of the barn, and we as community radiologists need to accommodate and modulate the trend and, in some states, follow the law with a tincture of professional and medically responsible acumen.
Automated breast ultrasound is a newer modality but not yet utilized on a routine basis in most practices. However, it stands on the threshold of greater importance as imagers become more comfortable with viewing standardized images obtained by automated breast ultrasound, which is not unlike viewing any other digitalized study such as CT or MRI. In time, as automated ultrasound becomes validated and more standardized, this alternative modality may play a significant role in evaluating dense breasts.
I believe that through the improved detection of appropriate asymmetries and architectural distortions, improved training, maintaining performance benchmarks within the prescribed limits, and utilization of the new technologies of breast tomosynthesis and automated breast ultrasound, breast imagers can move toward decreased recalls, decreased radiation exposure, decreased biopsy recommendations and hence fewer false positive studies which have negatively impacted on breast imaging as a whole. As a community radiologist, I believe that my work is making a positive impact on my patient’s lives. Not one week passes in which I don’t see a handful of women with variable breast density patterns and suspicious lesions, a few of which ultimately end up being diagnosed as malignant.
I see how both mammography and ultrasound, as well as the other tools of the breast imager’s armamentarium, allow me to save lives. Within two weeks of the initial preparation of this commentary, I diagnosed two small invasive carcinomas, yet each woman will get an excellent chance at a cure because those cancers were identified at a very small size, (</=1cm). Having worked in breast imaging for many years, I can assure my fellow clinicians that emotions for both the patient and practitioner can be formidable. It is humbling to know that in my imaging microcosm, which serves a large population, I can make a positive difference in women’s lives plying an imperfect tool.
I am fulfilled by applying the knowledge of my profession to the lives that I touch, but I also look forward to better imaging modalities and algorithms, and I actively endeavor to fine tune the skills required to better serve my patients.
References
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Int Med. 2009; 151(10):716-726.
- Sharpe RE, Levin DC, MD, Parker L, et al. The effect of the controversial US preventive services task force recommendations on the use of screening mammography. J Am Coll Radiol. 2013; 10(1): 21-24.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendation: science ignored. AJR Am J Roentgenol. 2011; 196(2): W112 – W116.
- Jørgensen KJ, Keen JD, Gøtzsche PC. Is mammographic screening justifiable considering its substantial overdiagnosis rate and minor effect on mortality? Radiology. 2011; 260(3): 621-627.
- Kopans DB, Smith RA, Duffy SW. Mammographic screening and “overdiagnosis.” Radiology. 2011; 260(3): 616-620.
- Tabar L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology. 2011; 260(3): 658 – 663.
- Kopans DB. Response to Dr Seidenwurm’s comments. J Am Coll Radiol. 2013; 10(5): 324.
- Carney PA, Sickles EA, Monsees BS, et al. Identifying minimally acceptable interpretive performance criteria for screening mammography. Radiology. 2010; 255(2): 354–361.
- Miglioretti DL, Ichikawa l. Smith RA et al. Criteria for identifying radiologists with acceptable mammography interpretation performance on basis of multiple performance measures. AJR Am J Roentgenol. 2015; 204(4): W486 - 491.
- Onega T, Smith M, Miglioretti DL, Carney PA, et al. Radiologist agreement for mammographic recall by case difficulty and finding type. J Am Coll Radiol. 2012; 9(11): 788-794.
- Rafferty EA, Park JM, Philpotts LE, et al. Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: results of a multicenter, multireader trial. Radiology. 2013; 266(1): 104-113.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013; 267(1): 47–56.
- Yaffe MJ, Mainprize JG. Risk of radiation-induced breast cancer from mammographic screening. Radiology. 2011; 258(1) 98-105.
- Hooley RJ, Greenberg KL, Stackhouse RM, et al. Screening US in patients with mammographically dense breasts: Initial experience with Connecticut Public Act 09-41. Radiology. 2012; 265(1): 59-69.
- D’Orsi CJ, Sickles EA. To seek perfection or not? That is the question. Radiology. 2012; 265(1): 9-11.
Citation
Ruocco M. Improving screening mammography: Perspective of a community radiologist. Appl Radiol. 2016;(9):26-29.
September 5, 2016