Imaging of mesenteric ischemia
Images
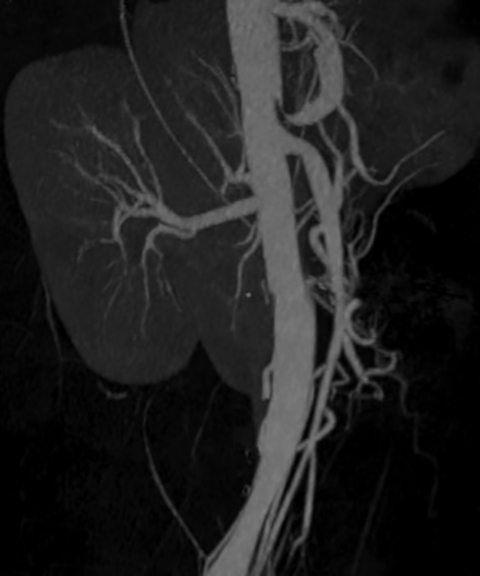

Mesenteric ischemia is a disease seen predominantly in the elderly that can be associated with considerable mortality if not detected before bowel infarction.1,2 Clinically, there are two subtypes of mesenteric ischemia: acute and chronic. Biphasic CT has become the gold standard in evaluating patients with suspected mesenteric ischemia.2 The modality provides rapid, noninvasive evaluation and minimizes the need for angiography and exploratory laparotomy. This review summarizes the role of CT and other imaging modalities in the evaluation of mesenteric ischemia.
CT evaluation
Computed tomography has been heralded as the primary imaging for the evaluation of mesenteric ischemia.2 The use of water as an oral contrast agent allows for improved visualization of the bowel wall and mucosa.3 Acquiring images in the arterial and venous phases (biphasic) allows for evaluating the arterial and venous mesenteric vessels as well for evaluating the bowel. Multidetector CT has further improved evaluation by enabling clinicians to obtain the level of detail needed to visualize 1 mm intravascular filling defects and air within the bowel wall. Distal mesenteric branches can be visualized with the rapid administration of intravenous contrast.3
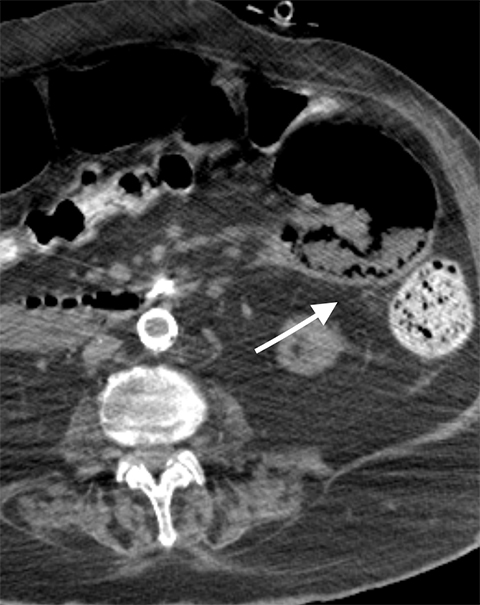
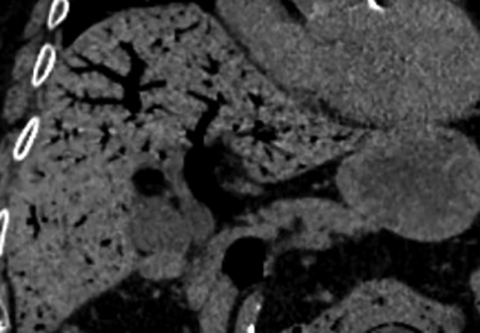
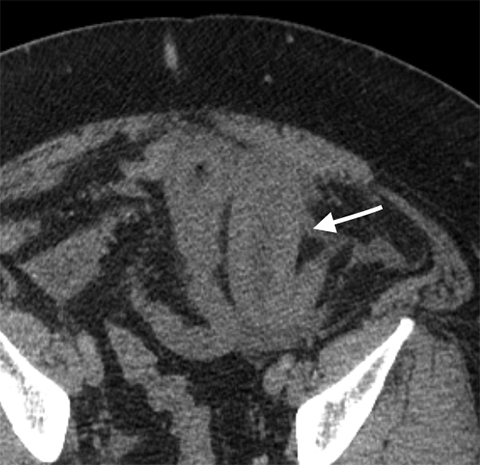
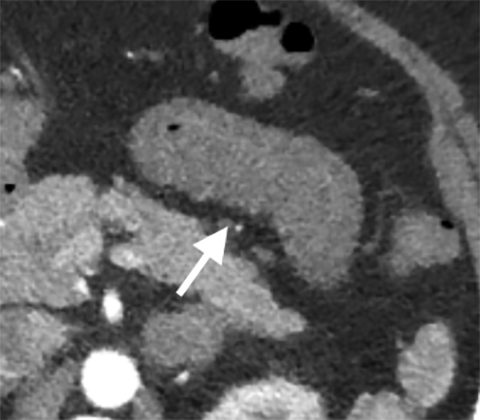
Multiple studies have also demonstrated the value of certain imaging findings, including pneumatosis intestinalis (Figure 1), isolated SMA occlusion, as well as celiac and inferior mesenteric artery occlusion with distal SMA disease (branch vessel occlusions or narrowing), arterial embolism, or venous gas (Figure 2). These findings have high specificity for acute mesenteric ischemia.2, 4, 5, 6 Bowel-wall thickening (Figure 3), focal lack of bowel wall enhancement, and venous thrombosis are slightly less specific (94%). The constellation of these findings on CT imaging yields high sensitivity and specificity for the diagnosis of acute mesenteric ischemia.7,8
Etiology of acute mesenteric ischemia
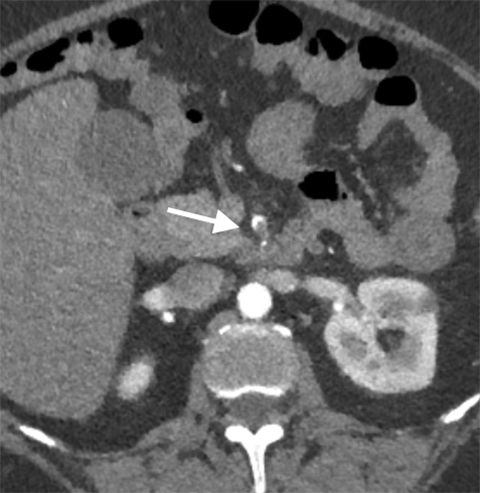
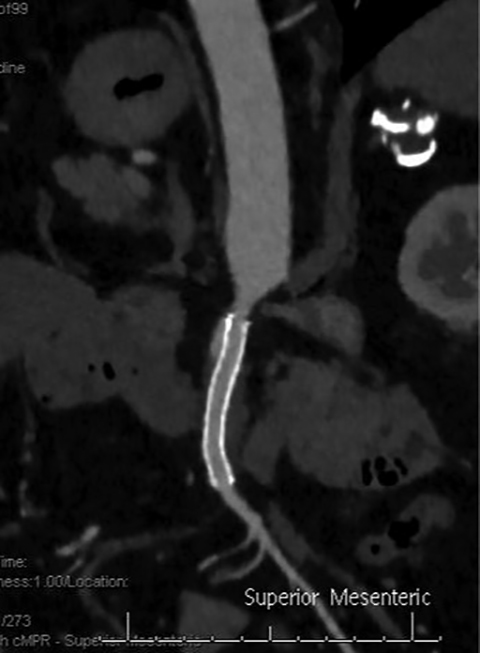
The etiology of acute mesenteric ischemia can be divided into four categories: arterial embolization, arterial thrombosis, mesenteric venous thrombosis, and non-occlusive, low-flow state.1 Although the patient’s clinical presentation may provide clues, the arterial-phase findings on CT may help distinguish between an embolism and a thrombosis. Commonly, an acute embolus is suspected when evidence of thrombosis appears elsewhere in the arterial tree, such as in the heart. In addition, an embolus tends to lodge within the most proximal 1-2 cm of the SMA, as opposed to thrombosis, which typically occurs at the vessel ostium (Figure 4).9 More specifically, 50% of thromboembolisms occur just distal to the middle colic branch, and 15% occlude the origin.10
Collateral mesenteric vessels on CT help determine the chronicity of an occlusion; when present, they suggest thrombosis secondary to chronic atherosclerotic stenosis. In contrast, patients with an acute thromboembolism are more likely to develop bowel ischemia, since they have not developed collaterals.11
Unlike arterial thromboembolism, venous thrombosis is unrelated to atherosclerosis. Hypercoagulable states, systemic processes, or an underlying inflammatory process from the abdominal organs are the etiologies for venous thrombosis.12 For example, pancreatitis or extrinsic compression by a pancreatic neoplasm are typical causes of SMV thrombosis. Mesenteric venous stasis is the third-leading cause of venous thrombosis and can occur as a result of a number of conditions, including Budd-Chiari, cirrhosis, or post-splenectomy physiology.12 Passive venous congestion of the end-organ eventually reduces arterial inflow to ultimately result in bowel infarction. Clinically, patients with venous thrombosis may present with a longer, more indolent course of symptoms (1-2 weeks). Because of venous congestion, bowel ischemia is more likely to demonstrate bowel-wall thickening, mucosal hyper-enhancement, and mesenteric edema, which can be either hypo- or hyperdense depending on whether edema or hemorrhage is prevalent.8 Conversely, bowel ischemia resulting from arterial thromboembolism is actually associated with bowel-wall thinning and no mucosal enhancement in the early stages.12,13 Ultimately, as bowel ischemia progresses to infarction, the conventional signs of reactive edema become progressively more apparent regardless of whether venous or arterial occlusion is the culprit (Figure 5).
Non-occlusive mesenteric ischemia does not demonstrate arterial filling defects or venous outflow occlusion and, therefore, has no typical imaging findings.14 The diagnosis relies on clinical presentation and is almost exclusively seen in acutely ill patients who are requiring pressor support. Occasionally, CT imaging may identify fewer distal branching vessels as a result of hypovolemia and spasm.15
Arterial phase imaging has been shown to influence patient care by demonstrating signs of acute mesenteric ischemia not seen on portal venous phase imaging.2 Arterial phase imaging can identify a vascular insult that maybe amenable to revascularization instead of bowel resection.16 Furthermore, some of these patients demonstrating vascular abnormalities prior to developing bowel ischemia can be treated before they develop the devastating downstream effects of infarction (Figure 6).17 Signs that suggest irreversible damage and warrant surgical resection of infarcted bowel include pneumatosis intestinalis and air within the portal venous system.2, 7, 8 A retrospective review has shown that more conservative treatment, including anticoagulation, can be implemented with success in cases of acute mesenteric ischemia without infarction caused by venous thrombosis diagnosed early with CT.17
Chronic mesenteric ischemia
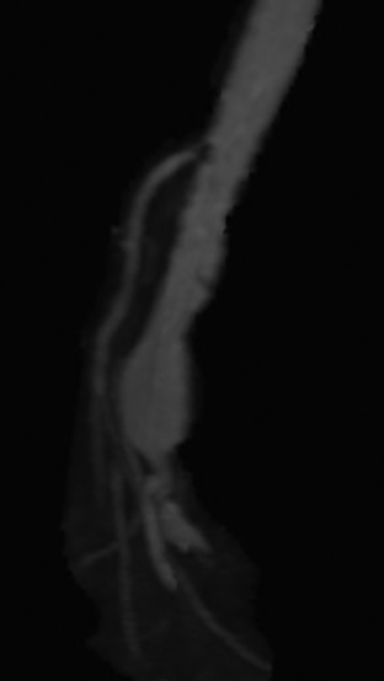
Chronic mesenteric ischemia (CMI) is caused by atherosclerotic disease of the mesenteric vessels.1 Its presentation is more indolent than acute mesenteric ischemia, typically weight loss and recurrent post-prandial abdominal pain when more than basal mesenteric blood flow is required for digestion.18 Given its chronic nature, CMI is commonly associated with development of rich collateral pathways. Three-dimensional reformatting in the coronal and sagittal plane aids in identification of collateralization and in evaluation of adequacy of compensated intestinal perfusion. As the presence of calcification is not specific to flow-limiting stenosis, vessel analysis has been shown to assess the extent of stenosis to the mesenteric vessels.18 A >70% stenosis of the celiac or superior mesenteric artery (SMA) is associated with a greater risk of having chronic mesenteric ischemia.19 Because of the rich mesenteric collateral network, a critical percentage of stenosis is usually not sufficient to cause chronic mesenteric ischemia and usually two of the three main mesenteric vessels must be compromised before symptoms develop.19
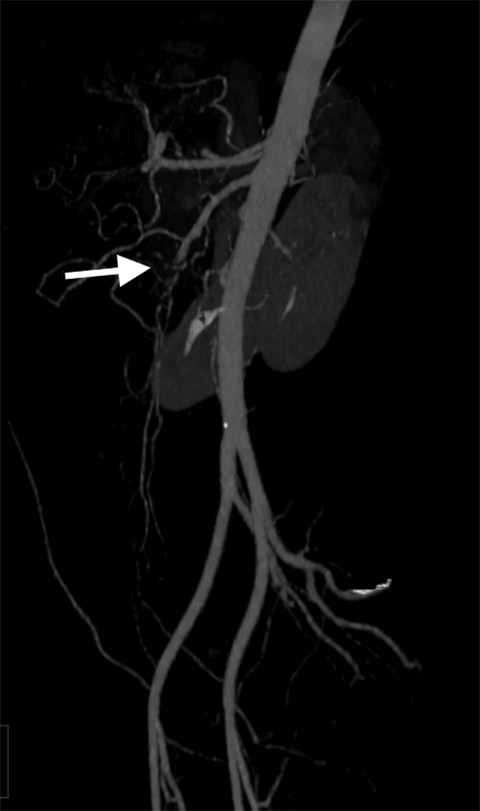
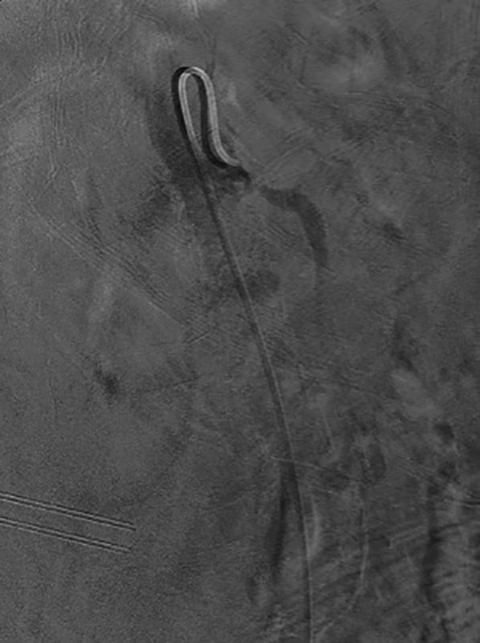
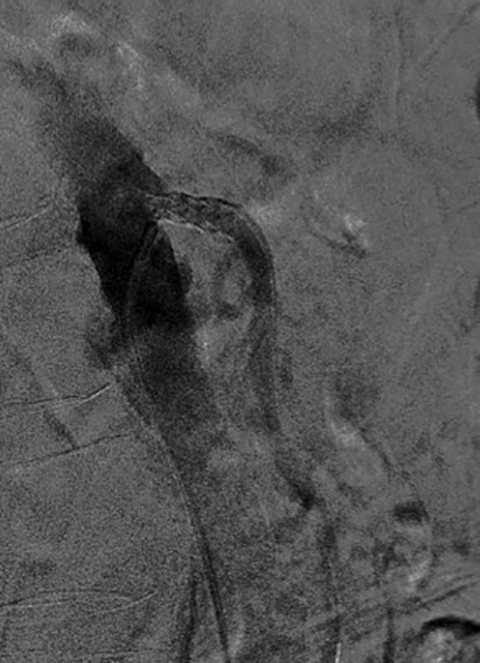
Median arcuate ligament syndrome is a less common cause of chronic mesenteric ischemia that may affect younger patients. The ligament is a fibrous arch which lies above the SMA and anterior to the aorta at the L1 level. Compression of the celiac trunk by the median arcuate ligament can cause epigastric pain and weight loss.20 Doppler ultrasound can be used to screen for median arcuate ligament syndrome by eliciting increased velocities suggestive of celiac artery stenosis that may be more pronounced during inspiration.20 CTA or MRA is the modality of choice for its evaluation. A focal stenosis of the celiac artery with a hooked appearance is the imaging hallmark of the disease (Figure 7).21 As it is a chronic process, collateral vessels between the celiac and superior mesenteric circulation, particularly involving the pancreaticoduodenal arcade, can be prominent. Unlike more common causes of chronic mesenteric ischemia, median arcuate ligament syndrome is not amenable to endovascular treatment.22
Mesenteric dissection
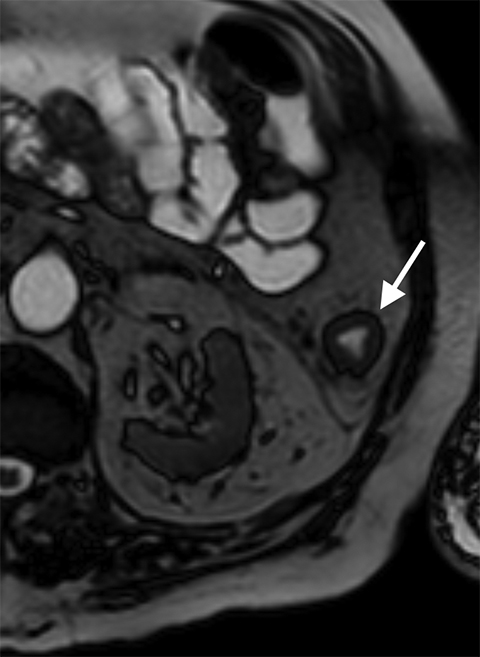
Arterial dissection of the mesenteric vessel is another rare cause of mesenteric ischemia. Most mesenteric dissections are found as extensions of abdominal aortic dissection; however, they can occur in isolation, precipitated by conditions affecting the vessel wall integrity such as fibromuscular dysplasia, vasculitis, cystic medial necrosis, Marfan’s syndrome, and Ehlers-Danlos disease, to name a few.23 Many dissections are asymptomatic, but when they limit vascular flow they may induce bowel ischemia. Findings include direct evidence of dissection, thrombosis of the false lumen, identification of an intimal flap, and enlargement of the artery and periarterial fat stranding (Figure 8).24
Mesenteric aneurysm
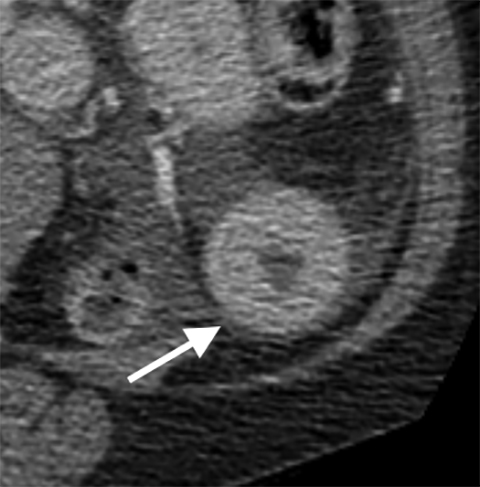
While rare, mesenteric aneurysms can also lead to mesenteric ischemia. SMA aneurysms are the third-most common type of visceral aneurysm after splenic and hepatic aneurysms. While splenic and hepatic aneurysms are usually related to atherosclerosis and risk of rupture and bleeding, mesenteric aneurysms are commonly due to subacute bacterial endocarditis, representing 58% of cases. Moreover, these aneurysms may not only rupture, but they also may threaten mesenteric flow by causing small-vessel occlusions due to distal emboli.25
Other imaging modalities
Computed tomography is the primary imaging modality used to evaluate mesenteric ischemia; ultrasound and MRI also play a role.26 Gray scale and Doppler ultrasound serve an important role in the screening, surveillance and follow-up of both mesenteric arterial and venous conditions.27 However, the yield of the ultrasound evaluation can vary due to body habitus, obscuring bowel gas, and operator capability.
Although MRA evaluation circumvents radiation or iodinated contrast, diminished spatial resolution can result in the overestimation of stenosis.28 Its longer acquisition time also limits its use in the emergent setting; ie, suspicion of acute mesenteric ischemia. However, in evaluating chronic mesenteric ischemia, MRA can provide functional information such as flow rates. While cardiac-triggered, respiratory-gated, 3D steady-state free precession sequences were developed for renal and mesenteric noncontrast MRA, 3D contrast-enhanced MRA is considered a more optimal examination. The contrast-enhanced, high-resolution 3D T1-weighted gradient-echo pulse sequence is the sequence used to evaluate for chronic mesenteric ischemia.28 Time-resolved MRI techniques can provide morphologic and hemodynamic flow information similar to digital subtraction angiography and thereby delineate AV fistulas, delayed portomesenteric venous filling and distal mesenteric flow in the evaluation of non-occlusive mesenteric ischemia.28
Angiographic evaluation and therapy
Angiographic evaluation of mesenteric ischemia is effective, although it is now reserved for intervention and treatment. Computed tomography has replaced angiography as a first line test to evaluate mesenteric ischemia, as it allows evaluation of the vessels and bowel and noninvasively identifies other reasons for the patient’s symptoms.2, 3, 7
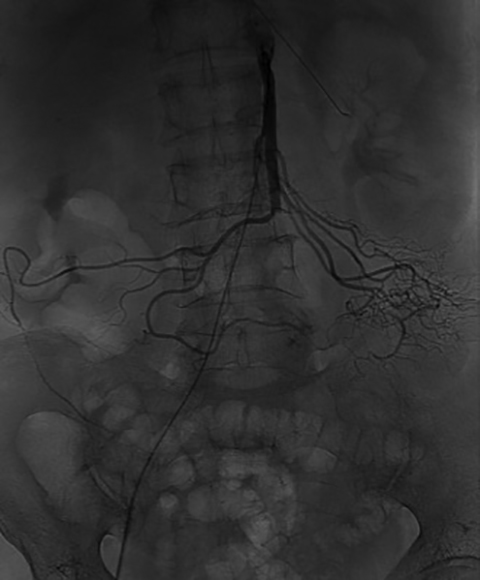
Endovascular therapy has become the primary treatment of patients with chronic mesenteric ischemia, allowing for safe revascularization with stent placement (Figure 9).29Additionally, aneurysms and dissections can be treated with endovascular techniques. The role of endovascular technique in the setting of venous thrombosis continues to expand. Patients with mesenteric venous thrombosis who don’t respond to anticoagulation may be treated endovascularly with transhepatic or transjugular approaches to remove thrombus and re-establish blood flow.30
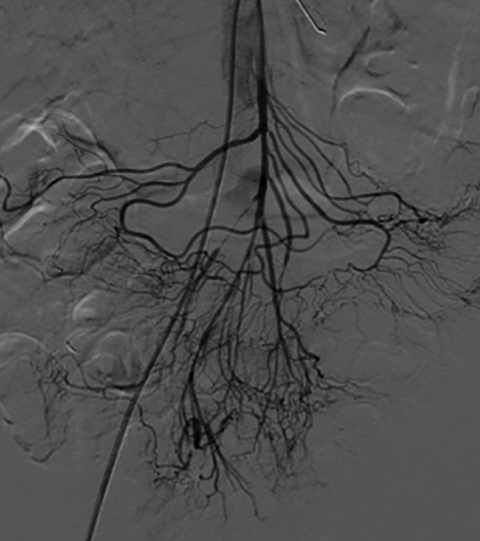
The use of endovascular techniques to treat acute mesenteric ischemia is usually reserved for patients who are not considered to be candidates for surgery. These therapies involve percutaneous thrombectomy techniques focusing on removing the acute embolus using suction (Figure 10).31
Follow up of mesenteric ischemia therapies
Computed tomography angiography remains the modality of choice with respect to primary evaluation and follow up of mesenteric ischemia patients, including those who have undergone surgical embolectomy, thrombectomy, bypass graft placement, endarterectomy, surgical release of median arcuate ligament syndrome, mechanical thrombectomy, angioplasty, catheter-directed thrombolysis, fenestration of the dissection membrane, stent placement and endoluminal stent-graft placement.32
Conclusion
An uncommon disease, mesenteric ischemia carries significant morbidity and mortality if not identified early in its course. Computed tomography has become the initial and best modality to assess for acute mesenteric ischemia because of its ability to evaluate not only the vessels but also the extent of bowel ischemia and/or infarction. The modality also identifies the cause of mesenteric ischemia and allows for prompt and proper triage and therapy. In the setting of chronic mesenteric ischemia, CT identifies the extent of vascular disease to select those patients who may require revascularization.
References
- Clair DG, Beach JM, et al. Mesenteric ischemia. N Engl J Med. 2016; 374(10):959-968.
- Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology. 2010;256(1):93-101.
- Horton KM, Fishman E, et al. Multidetector CT angiography in the diagnosis of mesenteric ischemia. Radiol Clin North Am. 2007;45(2): 275-288.
- Costa AF, Chidambaram V, Lee JJ, et al. Multidetector computed tomography of mesenteric ischaemia. Insights Imaging. 2014;5(6): 657–666.
- Levy AD. Mesenteric ischemia. Radiol Clin North Am. 2007;45(3):593–599.
- Duran R, Denys AL, Letovanec I, et al. Multidetector CT features of mesenteric vein thrombosis. Radiographics. 2012;32(5):1503–1522.
- Kirkpatrick I, Kroeker MA, Greenber HM, et al. Biphasic CT with mesenteric CT angiography in the evaluation of acute mesenteric ischemia: initial experience. Radiology. 2003; 229(1): 91-98.
- Horton K, Fishman E, et al. CT Angiography of the mesenteric circulation. Radiol Clin North Am. 2010;48(2): 331-345.
- Walker TG. Mesenteric ischemia. Semin Intervent Radiol. 2009;26(3): 175–183.
- Oldenburg WA, Lau LL, Rodenberg TJ, et-al. Acute mesenteric ischemia: a clinical review. Arch. Intern. Med. 2004;164 (10): 1054-62.
- van Petersen AS, Kolkman JJ, Meerwaldt R,et al. Mesenteric stenosis, collaterals, and compensatory blood flow. J Vasc Surg. 2014;60(1):111-119.
- Dhatt HS, Behr SC, Miracle A, et al. Radiological evaluation of bowel ischemia. Radiol clin North Am. 2015;53(6): 1241–1254.
- Reginelli A, Iacobellis F, Berritto D, et al. Mesenteric ischemia: the importance of differential diagnosis for the surgeon. BMC Surg. 2013;13 Suppl 2:S51.
- Bourcier S, Oudjit A, Goudard G, et al. Diagnosis of non-occlusive acute mesenteric ischemia in the intensive care unit. Ann Intensive Care. 2016;6(1):112.
- Woodhams R, Nishimaki H, Fujii K, et al. Usefulness of multidetector-row CT (MDCT) for the diagnosis of non-occlusive mesenteric ischemia (NOMI): assessment of morphology and diameter of the superior mesenteric artery (SMA) on multi-planar reconstructed (MPR) images. Eur J Radiol. 2010;76(1):96-102.
- Arthurs Z, Titus J, Bannazadeh M, et al. A comparison of endovascular revascularization with traditional therapy for the treatment of acute mesenteric ischemia. J Vasc Surg. 2011;53(3):698-704; discussion 704-5.
- Di Minno M, Milone F, Milone M, et al. Endovascular thrombolysis in acute mesenteric vein thrombosis: A 3-year follow-up with the rate of short and long-term sequaelae in 32 patients. Thromb Res. 2010;126(4):295–298.
- Horton K, Fishman E. Multi–detector row CT of mesenteric ischemia: can it be done? RadioGraphics 2001; 21(6):1463–1473.
- Hohenwalter E. Chronic mesenteric ischemia: diagnosis and treatment. Semin Intervent Radiol. 2009; 26(4): 345–351.
- Tembey R, Bajaj AS, Wagle PK, et al. Real-time ultrasound: Key factor in identifying celiac artery compression syndrome. Indian J Radiol Imaging. 2015;25(2):202-205.
- Horton KM, Talamini MA, Fishman EK, et al. Median arcuate ligament syndrome: evaluation with CT angiography. Radiographics. 2005;25:1177-1182.
- You JS, Cooper M, Nishida S, et al. Treatment of median arcuate ligament syndrome via traditional and robotic techniques. Hawaii J Med Public Health. 2013; 72(8): 279–281.
- Nath A, Yewale S, Kousha M, et al. Spontaneous isolated superior mesenteric artery dissection. Case Rep Gastroenterol. 2016;10(3):775-780.
- Suzuki S, Furui S, Kohtake H, et al. Isolated dissection of the superior mesenteric artery: CT findings in six cases. Abdom Imaging. 2004;29(2):153-157.
- Nosher J, Chung J, Brevetti LS, et al. Visceral and renal artery aneurysms: A pictorial essay on endovascular therapy. RadioGraphics 2006; 26(6): 1687-1704.
- Shetty A, Mellnick VM, Raptis C, et al. Limited utility of MRA for acute bowel ischemia after portal venous phase CT. Abdom Imaging. 2015;40(8):3020-3028.
- Reginelli A, Genovese E, Cappabianca S, et al. Intestinal ischemia: US-CT findings correlations. Crit Ultrasound J. 20135(Suppl 1):S7.
- Hagspiel K, Flors L, Hanley M, et al. Computed tomography angiography and magnetic resonance angiography imaging of the mesenteric vasculature. Tech Vasc Interv Radiol. 2015; 18(1):2–13.
- Kärkkäinen J, Acosta S. Acute mesenteric ischemia (Part II) – Vascular and endovascular surgical approaches. Best Pract Res Clin Gastroenterol. 2017;31(1):27-38.
- Yang S, Fan X, Ding W, et al. Multidisciplinary stepwise management strategy for acute superior mesenteric venous thrombosis: an intestinal stroke center experience. Thromb Res. 2015; 135: 36–45.
- Ryer E, Kalra M, Oderich GS, et al. Revascularization for acute mesenteric ischemia. J Vasc Surg. 2012; 55: 1682–1689.
- Shih P, Angle JF, Leung DA, et al. CTA and MRA in mesenteric ischemia: Part 2, normal findings and complications after surgical and endovascular treatment. AJR Am J Roentgenol.
Citation
V T, P I, CS P.Imaging of mesenteric ischemia. Appl Radiol. 2018; (2):13-18.
February 5, 2018