Imaging of lung transplantation
Dr. Ravenel is an Associate Professor of Radiology, Department of Radiology, Medical University of South Carolina, Charleston, SC. Dr. McAdams is a Professor of Radiology, Department of Radiology, Duke University Medical Center, Durham, NC.
In 1983, lung transplantation became a clinical reality and the number of procedures performed is steadily increasing. Approximately 1400 lung transplants are performed worldwide each year, 1 and there are now >17,000 patients in the International Society for Heart and Lung Transplantation (ISHLT) registry. 2 Although the procedure itself remains limited to various regional centers, as more and more patients with lung transplants return to their home communities, it will be important for radiologists to appropriately image as well as recognize complications that can result from the procedure. The purpose of this article is to discuss the important imaging considerations related to lung transplantation, particularly those that are expected to develop outside the transplant center.
Overview of lung transplantation
For many patients with end-stage lung disease, lung transplant is the only option available, and it offers the chance for their quality of life to be significantly improved. In the adult, the vast majority of lung transplants are performed for 4 conditions: chronic obstructive pulmonary disease (COPD) (39%), idiopathic pulmonary fibrosis (17%), cystic fibrosis (16%), and alpha-1-antitrypsin deficiency (9%). 2 Whenever possible, bilateral lung transplants are preferred; however, in cases of pulmonary fibrosis and selected cases of emphysema, single-lung transplants are acceptable and help to distribute needed organs to a larger population of critically ill patients. Because of the risk of infection and cross-contamination, unilateral lung transplants are contraindicated in patients with cystic fibrosis and bronchiectasis. Overall, an equal number of single- and double-lung transplants have been performed annually since 1995. 2
The benefit of lung transplantation comes in the form of both survival and quality of life. 1 However, the benefit is not realized until 3 to 6 months following transplant and comes with up-front risk. Even in experienced transplant programs, operative mortality rates may be as high as 8% and 3month survival is approximately 84%. 2 From 1992 to 2003, overall survival at 1, 3, 5, and 10 years was 74%, 58%, 47%, and 24%, respectively, with the average life expectancy following transplant just more than 3 years. In general, patients with COPD, emphysema, and cystic fibrosis fare better than those who received transplants for other conditions. 2
Preoperative imaging considerations
Recipient considerations
The preoperative imaging evaluation is designed to assess the transplant candidate for suitability and to select the most appropriate hemithorax in those considered for single-lung transplantation. To that end, these patients will undergo chest radiography, computed tomography (CT), and quantitative perfusion scintigraphy, at a minimum. Important considerations include the presence or absence of suspicious lung nodules, the presence or absence of pleural disease, or the presence of any other anatomic features that may impact surgical technique and differential perfusion. 3 The presence of mycetomas, while not an absolute contraindication, has been associated with a much higher mortality rate. 4 Preferably, the lung with the worse function will be transplanted. In 11% of cases, CT findings will change the lung selected for transplantation when compared with chest radiography. 5 As lung cancer is an absolute contraindication to transplant, suspicious lesions on CT should be evaluated thoroughly prior to transplant. The rate of cancer detection is similar to that of screening a high-risk population (1% to 2%). 5 In young patients with cystic fibrosis who are being considered for bilateral lung transplantation, CT has limited utility and has not been shown to change the surgical approach and, thus, may be omitted in this limited circumstance. 6
Donor considerations
Inherent difficulties in the management of potential organ donors following brain death put a strain on the number of available organs. Fortunately, a more aggressive approach to donor selection and extended acceptance criteria has been successful at increasing available donor organs without adversely affecting transplant outcome. 7 In an optimal situation, donors should have a normal chest radiograph and no evidence of chest trauma or aspiration. However, the presence of improving air-space opacities, contusion, pneumothorax, or pleural effusion does not appear to impact the postoperative course. Worsening or persistent air-space opacities in a potential donor remains a contraindication to using the lungs for transplant. 7
Imaging in the immediate postoperative period
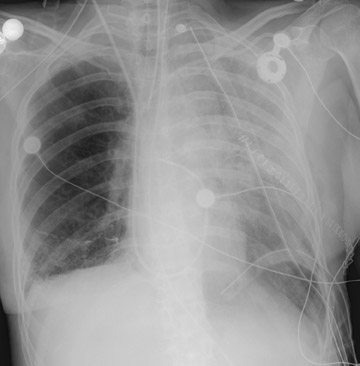
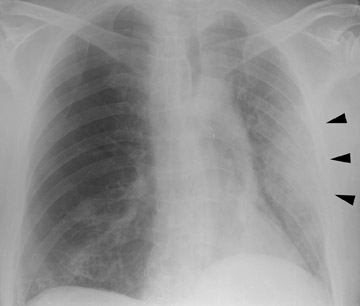
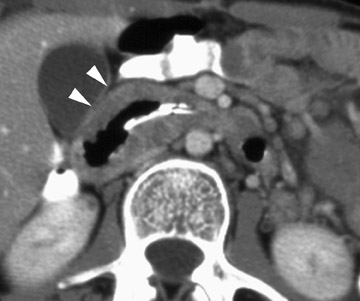
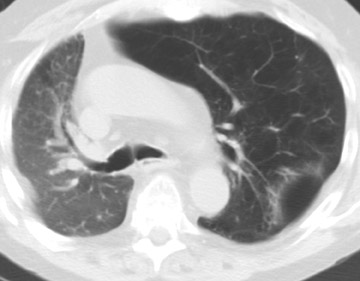
In the postoperative period, there are 3 major complications: Reperfusion edema, acute rejection, and infection. Reperfusion edema (Figure 1) is the most frequent immediate postoperative complication that occurs within several hours up to 48 hours following the procedure and is characterized radiographically by mixed interstitial and air-space opacities in the transplanted lung(s) and by small pleural effusions. 8 Risk factors include poor organ preservation, prolonged ischemic time, unsuspected donor pathology such as contusion and aspiration, interrupted lymphatic supply, and cytokine-mediated injury. 9-11 Treatment consists of diuresis and mechanical support. With appropriate therapy, the condition generally improves over the next 24 to 48 hours and resolves over 7 to 10 days, rarely contributing to morbidity and mortality by itself.
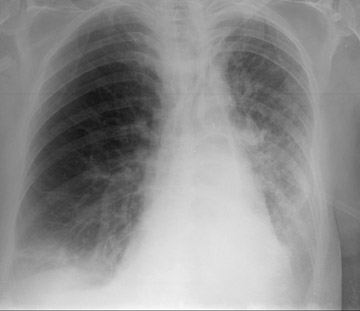
Acute rejection has a radiographic appearance similar to reperfusion injury (Figure 2)-that is, mixed interstitial and air-space opacities with associated pleural effusions. 12 Therefore, the timing of edema is important, as acute rejection usually does not develop until 7 to 10 days following the procedure at the earliest. Reperfusion edema and acute rejection are best distinguished by the temporal relationship to the transplant procedure. CT is of limited utility in the diagnosis. Ground-glass opacities, septal lines, and pleural effusions are often present but non-specific. 11,13 Most patients will have at least 1 episode of acute rejection in the first year following transplant.
It is important to recognize that infections may occur at any time during the postoperative period and may coexist with edema and acute rejection. Infection has a major impact on survival and is a primary cause of postoperative mortality in up to 50% of cases. 14 The radiographic manifestations are varied and reflect the nature of the organism and the host response. Bacterial organisms (usually Staphylococcus , Pseudomonas , and other gram-negative organisms) are the most frequent cause of infection in the immediate postoperative period. 15
Because of impaired fluid clearance through the pleura, effusions (frequently transudative effusions) occur in almost every case. 16 While not all effusions require drainage, small-bore catheter drainage is usually successful. 17 Occasionally, effusions may require protracted drainage to effect a complete response.
Imaging of complications after discharge
Infection
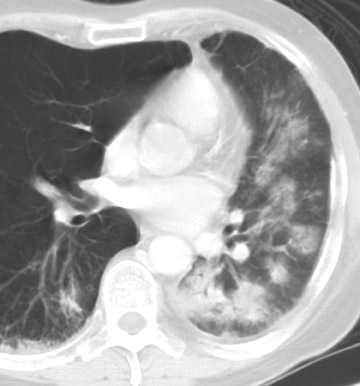
Although bacterial infections predominate in the first month following lung transplant, viral infections, particularly cytomegalovirus (CMV), become more frequent in the second and third months and continue thereafter. While the exact incidence of CMV pneumonia after lung transplant is unclear, it is estimated to occur in up to 86% (range 35% to 86%) of transplant recipients, even with appropriate prophylaxis. Mortality for CMV pneumonia ranges from 2% to 12%. 18 Also, CMV infection remains an important cause of bronchiolitis obliterans syndrome (BOS), described later. 18,19
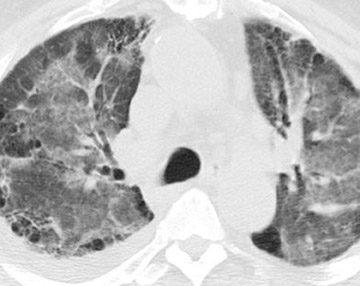
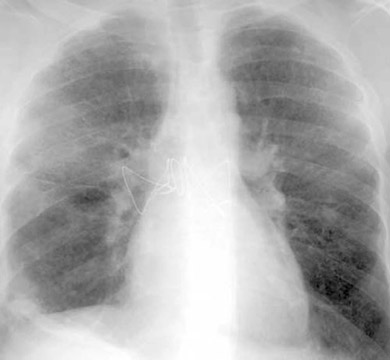
The radiographic manifestations (Figure 3) have been described in small series and include heterogeneous air-space opacities, ground-glass opacities, nodules, septal lines, and pleural effusion in relatively similar frequency. 15 In unilateral lung transplant recipients, infection usually occurs in the transplanted lung. Other viral organisms (including influenza, parainfluenza, respiratory syncytial virus, and adenovirus) have also been described. Radiographs are essentially normal in half of the cases. In cases with abnormal findings, nonspecific heterogeneous opacities are the rule. 20
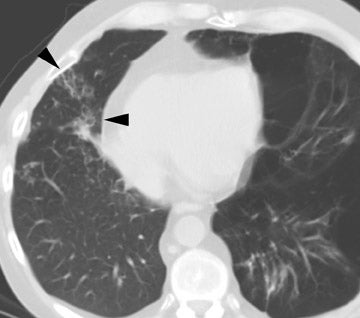
Fungal infections (mainly with Aspergillus species) may also occur at any time. Aspergillus species cause infection in 6% of cases 21,22 and appear to have a higher incidence in patients with cystic fibrosis and in patients who had a single-lung transplant for COPD. 21,23 Unfortunately, it can be quite difficult to separate colonization from true infection. In the majority of cases, the infection is either a tracheobronchitis or an anastomotic infection, whereas invasive pulmonary infections and disseminated disease are less frequent. 21 Complications of Aspergillus infection in the airways include both stenosis and anastomotic dehiscence. Overall mortality is approximately 50%. 21
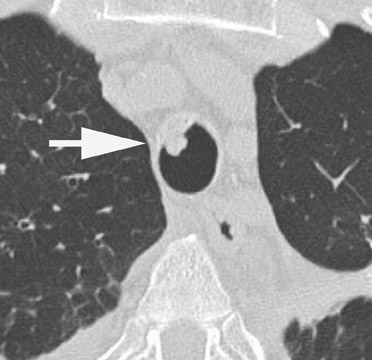
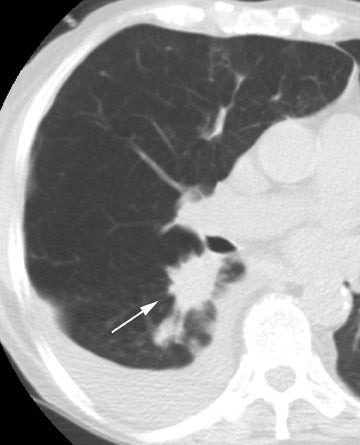
Radiographic findings are primarily based on the site of disease. Chest radiographs are generally normal in tracheobronchitis. CT, including virtual bronchoscopy, may reveal surface mucosal irregularities (Figure 4) and be useful to document stenosis and dehiscence in certain circumstances. 24 However, since the diagnosis must be established by bronchoscopy, there is no primary role for CT in this regard. Nodules and focal consolidation are the primary manifestations of invasive disease both with chest radiographs and CT, 15 and findings are similar to those of invasive Aspergillus infection in other settings.
Rejection
Acute rejection, described previously, may occur at any time, although the ma-jority of episodes occur within the first year (Figure 5). Chronic rejection, also termed bronchiolitis obliterans syndrome (BOS), is quite common and probably develops in all transplant patients given sufficient time. Risk factors include frequent or severe bouts of acute rejection, infection, and gastroesophageal reflux. 25-27 The diagnosis is established by pulmonary function testing and graded by criteria developed by the ISHLT (Table 1). 28 Bronchiolitis obliterans syndrome remains the most significant barrier to long-term survival, and, unfortunately, it is difficult to establish the diagnosis early.
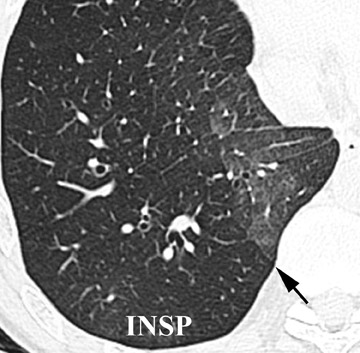
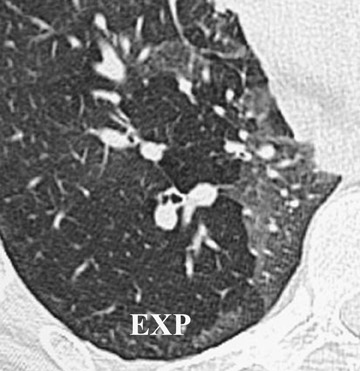
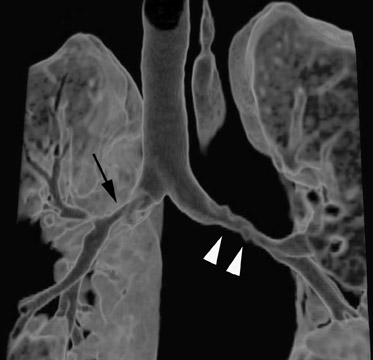
Initially, it was hoped that high-resolution CT (HRCT) would provide early clues to BOS and predict those who are at risk for development of BOS, thus allowing for earlier treatment. Chief among possible clues was the presence of air trapping on expiratory CT; various strategies have been developed to document air trapping (Figure 6). Early reports put the sensitivity of air trapping on expiratory HRCT between 75% to 90%. 29-31 With the revision of the ISHLT grading system, expiratory CT has been found to be less sensitive than had been previously thought, 32 and this undoubtedly reflects the greater reliance on more subtle physiologic changes for the diagnosis. When air trapping is present, the specificity of CT has been found to be quite good in several series. 30,32 Other CT findings of BOS include mosaic attenuation, cylindrical bronchiectasis (Figure 7), and bronchial wall thickening. These features have been shown to be weak correlates of disease severity, however. 33
Airway complications
With refinements in surgical technique, bronchial complications are less frequent. Complete dehiscence is quite rare and requires surgical intervention. More commonly (but still rarely) an incomplete dehiscence will occur. Chest radiographs may reveal an expanding pneumothorax or pneumomediastinum but most often do not reveal acute abnormalities. 34 At CT, focal defects of the bronchial wall and perianastomotic air collections may be seen. 35,36 The majority of incomplete dehiscence can be managed conservatively, and some may heal spontaneously.
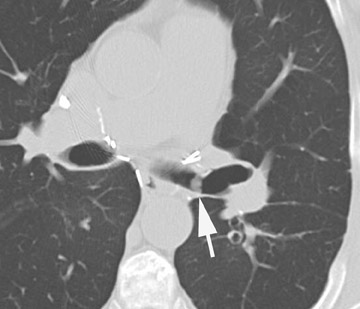
An important mimicker of dehiscence is the telescoping bronchus. This technique results in an end-to-end anastomosis of the posterior membranous portion of the bronchus and invagination of the cartilaginous portion of the smaller bronchus into the larger bronchus. 37 The telescoping segment, therefore, arises from either the donor or the recipient. On axial CT images, the telescoped segment may have the appearance of a bronchial wall defect or an apparent extraluminal gas collection. 38 Thin sections with oblique coronal reformatted images are valuable in separating the telescoping bronchus from dehiscence. 38
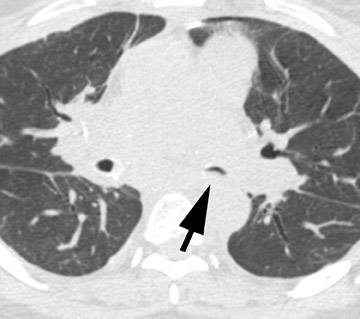
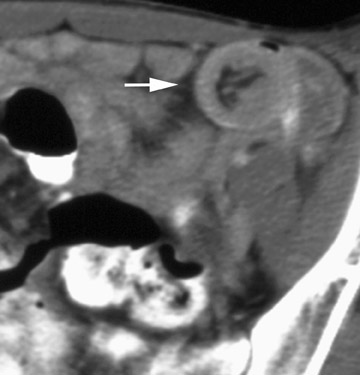
Stenosis at the anastomotic site is a more common occurrence (Figure 8). Both CT and virtual bronchoscopy are accurate for stenosis detection 24 ; however, as fiberoptic bronchoscopy is required for definitive diagnosis and management, the utility of CT is limited. Management strategies include debridement of granulation tissue or stent placement.
Posttransplant lymphoproliferative disorder
Posttransplant lymphoproliferative disorder (PTLD) is an uncommon complication in transplant recipients and occurs in <6% of patients. 39 Posttransplant lymphoproliferative disorder covers a wide spectrum of lymphoid proliferation that ranges from hyperplastic colonies of polyclonal B-cells to aggressive monoclonal high-grade lymphoma. A slight majority occurs in the first year after transplant. Intrathoracic disease is most common in the first year, whereas late development of PTLD is associated with extra-thoracic sites of disease (Figure 9). 39 The most important risk factors include immunosuppression with cyclosporine and Epstein-Barr virus infection. The most common findings, both at chest radiography and CT, are multiple well-circumscribed pulmonary nodules with or without a ground-glass halo. 40,41 Other reported radiographic findings include hilar and mediastinal adenopathy, parenchymal consolidation, and pleural masses. Disease outside the thorax most frequently occurs in the gastrointestinal tract but may also be seen in the skin, oropharynx, and solid organs. 39
Other complications
Lung cancer develops in the native lung of single-transplant recipients in approximately 1% of cases, with slightly higher rates overall for those with emphysema and pulmonary fibrosis (Figure 10). 42 In a multicenter series, 9 of 24 lung cancers (38%) were stage I. 42 Recurrence of the primary disease has been described with many conditions, including sarcoidosis, lymphangio-leiomyomatosis, Langerhans cell histiocytosis, talc granulomatosis, diffuse panbronchiolitis, and alveolar proteinosis. Although the overall incidence of recurrence is approximately 1% of all transplant recipients, it is a relatively common phenomenon in patients with sarcoidosis (35% recurrence rate). 43 Disease recurrence often occurs without overt radiographic findings or is found incidentally at transbronchial biopsy. When radiographic findings are present, they generally follow the typical features of the underlying disease. In sarcoidosis, rather than adenopathy, fine interstitial opacities and miliary nodules are the most common manifestations of recurrent disease (Figure 11). 43
Complications in the native lung should not be overlooked in single-lung transplant recipients. In up to 50% of cases of emphysema, progressive hyperinflation develops (Figure 12). This, in turn, can have adverse effects on the transplanted lung through compression and an increase in pulmonary vascular resistance. 44,45 In one transplant center, native lung complications (aside from hyperinflation) occurred in 15%, most often infectious, and caused serious morbidity and mortality in 70% of patients. 44,45
Conclusion
Lung transplantation is increasingly utilized in the management of end-stage pulmonary diseases. Practicing radiologists who are not affiliated with transplant centers need to be aware of the various conditions and complications related to lung transplantation so that appropriate management can be implemented rapidly.
