GE Invenia Automated Breast Ultrasound system updated
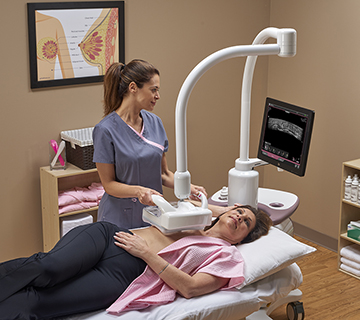
 GE Healthcare has launched the Invenia Automated Breast Ultrasound (ABUS) 2.0 system in the United States. This U.S. Food and Drug Administration (FDA)-approved supplemental breast screening technology is specifically designed to detect cancer in dense breast tissue.
GE Healthcare has launched the Invenia Automated Breast Ultrasound (ABUS) 2.0 system in the United States. This U.S. Food and Drug Administration (FDA)-approved supplemental breast screening technology is specifically designed to detect cancer in dense breast tissue.
The Invenia ABUS 2.0 incorporates features designed to customize the breast examination based on a patient’s body. The cSound™ Imageformer, a software-based graphics processor, provides a reproducible and operator-independent acquisition method to achieve consistent, high quality results. cSound imaging allows significantly more data to be collected and used to create every image, according to GE Healthcare.
The Reverse Curve™ transducer follows the natural contour of the breast, providing patient comfort, thorough contact and helping ensure comprehensive coverage. The 15 cm large field-of-view transducer is easy to position and maintains even compression while scanning. Since no two women are identical, exams can be customized with programmable scan protocols, adjustable scan depths and compression levels. A sonographer also has the ability to shorten scan time once breast tissue acquisition is complete by pushing a single button
