Endovascular stent placement in femoro-popliteal arterial occlusive disease
Images



Dr. Drescher is a Clinical Assistant Professor of Radiology and Interventional Radiology, Medical College of Wisconsin, Great Lakes Radiologists, Milwaukee, WI.
Since the pioneering work by Dotter and Judkins 1 more than 3 decades ago, percutaneous transluminal angioplasty (PTA) is still considered the gold standard for endovascular treatment of patients with symptomatic femoropop-liteal arterial occlusive disease (FPAOD). Numerous studies have shown the safety and the durability of this procedure. 2 With the introduction of improved metallic stents, covered stents, and coated stents, percutaneous treatment options for FPAOD have expanded. This article will provide an overview of the current status of stent placement in FPAOD.
Clinical indications and considerations for treatment of FPAOD
Clinical indications for treatment of FPAOD include intermittent claudication, rest pain, and tissue loss, such as ulceration and gangrene. A thorough clinical examination is essential prior to undertaking PTA or stent placement. A widely used clinical system is the Rutherford's classification of chronic limb ischemia 3 (Table 1). Noninvasive hemodynamic studies should be performed prior to intervention, including resting ankle brachial indices (ABI), segmental limb and toe pressure measurements, and pulse volume recordings of the lower extremities. Although these noninvasive evaluations correlate with the severity of ischemic symptoms, they often fail to differentiate between stenotic and occlusive lesions. Furthermore, in diabetic patients, who represent a large group of patients in need of FPAOD intervention, the presence of calcified, non-compressible arteries might render interpretation of noninvasive studies difficult. In these cases, color duplex imaging can be used to differentiate stenotic from occlusive lesions and to grade the severity of a fiow-limiting lesion using measurements of peak systolic velocities. These findings have been favorably correlated with angiographic findings. Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) are being used more frequently for preprocedure evaluation.
The indications for intervention remain controversial. A patient with mild intermittent claudication might not require any intervention; in such patients, a program of smoking cessation, progressive walking exercise, and control of diabetes, hyperlipidemia, hypertension, or hyperhomocystinemia can lead to symptom improvement. A recent study randomizing patients with claudication to PTA versus medical therapy (aspirin, walking exercise, and smoking cessation) found no significant difference in clinical outcome as indicated by maximum walking distance, treadmill exercise, ABI, and quality of life. 4 On the other hand, patients with severe lifestyle-limiting claudication, patients failing conservative treatment, and patients with limb-threatening ischemia (rest pain or tissue loss) should be considered for intervention.
Classification and patient selection
The technical success, durability, and morbidity of percutaneous intervention correlate directly with clinical and anatomic factors. Patients presenting with critical limb ischemia have a significantly lower patency rate after PTA of FPAOD compared with patients treated for claudication. Diminished crural outfiow and the high prevalence of diabetes mellitus in this patient population are likely factors for these results. The most important anatomic factor for determining outcome of percutaneous intervention in the femoropopliteal segment is lesion morphology. The Trans-Atlantic Inter-Society Consensus (TASC) has stratified types of lesions based on the length of the lesion, the angiographic appearance of the lesion, and the presence of a complete occlusion rather than a stenosis (Table 2). 2
After evaluation of the runoff vessels, the crucial question remains whether to treat patients by endovascular means or with surgery. The arguments for balloon angioplasty or stent placement in FPAOD include the low risk of the procedure and the preserved potential for bypass surgery. The argument for surgical bypass is the potential for improved patency, particularly when a saphenous vein is used rather than synthetic material. Unfortunately, patients with FPAOD often have coronary artery disease, thus, preserving the saphenous veins for coronary bypass grafting should be a consideration as well. The only long-term prospective randomized study comparing bypass surgery with PTA showed no significant difference in patency rate or limb salvage. 5 A meta-analysis including decision-making tools and cost-effectiveness ratios comparing these two treatment options suggested that bypass surgery was most effective in patients with critical limb ischemia and arterial occlusion. 6 In general, it appears that surgery is more successful in patients with long-segment disease, complex arterial occlusions, and tibial multivessel disease and the two methods should be viewed as complementary. Treatment is often based on an individual patient basis and on the local availability of experts in these fields.
Technique of PTA and stent placement in FPAOD
The initial step to FPAOD intervention often is a lower extremity angiogram with the goal to determine whether surgery or intervention should be performed. The initial arterial access is contralateral to the ischemic limb in order to keep the options open for antegrade or retrograde puncture of the symptomatic side. To treat complex or distal lesions, antegrade puncture of the femoral artery on the affected side should be considered. This approach will minimize the need for exchange length wires, and for long-shafted catheters, balloons, and stents, and will allow better control of catheters and wires. Antegrade punctures are associated with a higher complication rate and make it difficult to treat proximal superficial femoral artery lesions. If the intervention is to be performed from the contralateral access, an up-and-over sheath should be placed. The sheath tip should be placed over the aortic bifurcation into the contralateral common femoral artery. This typically requires a 35- to 45-cm sheath length.
A 5F sheath is sufficient for PTA balloons between 4 and 6 mm and PTA monorail balloons up to 7 to 8 mm. For stent placement, a larger sheath size is often required (Tables 3 through 5). For recanalization of occlusions and complex stenoses, hydrophilic guidewires (0.014- to 0.035-inch) and 3F to 5F catheters are often helpful. After crossing the stenosis or occlusion, intravenous heparin (40 to 60 U/kg) and, often, intra-arterial vasodilators (nitroglycerin 100 to 200 µg) are administered.
In most cases, PTA remains the initial treatment. The balloon should be slightly oversized to the vessel size. There are several ways to measure the vessel, including using a ruler on the angiographic table adjacent to the affected limb or using a reference catheter. Balloon infiation is performed for 30 to 60 seconds, and prolonged infiation (2 to 3 minutes, 4 to 6 atm) can be helpful to "tack" a resilient dissection fiap. It is generally accepted that technical success is achieved if <30% residual stenosis is present on control angiography. Also, pressure measurements before and after PTA can be performed. A mean pressure gradient of 10 mm Hg without vasodilation and 20 mm Hg with vasodilation are believed to demonstrate hemodynamic significance. Vasodilation can be achieved by injection of 50 to 100 µg of nitroglycerin or 10 to 30 mg of papaverine.
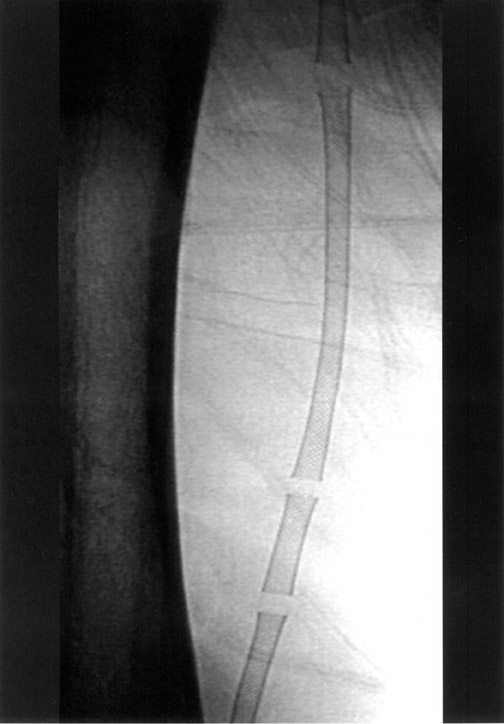
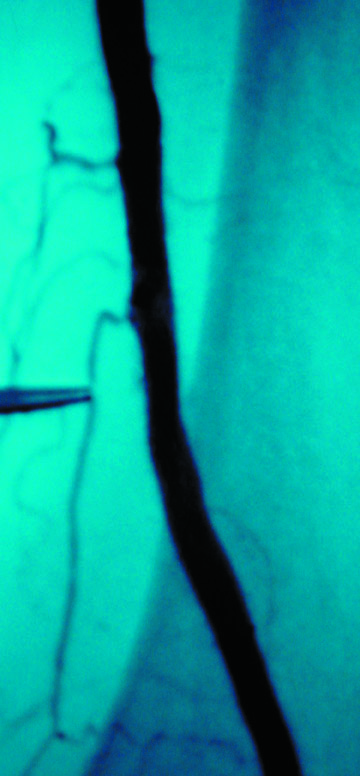
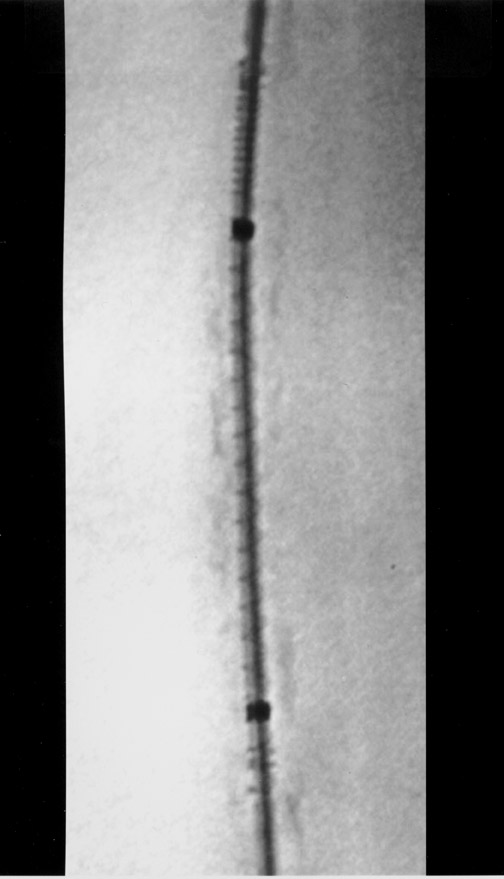
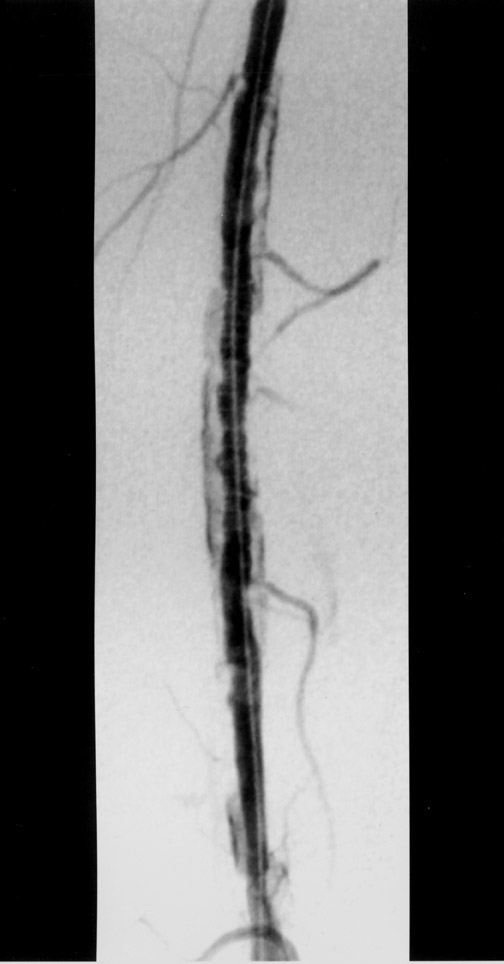
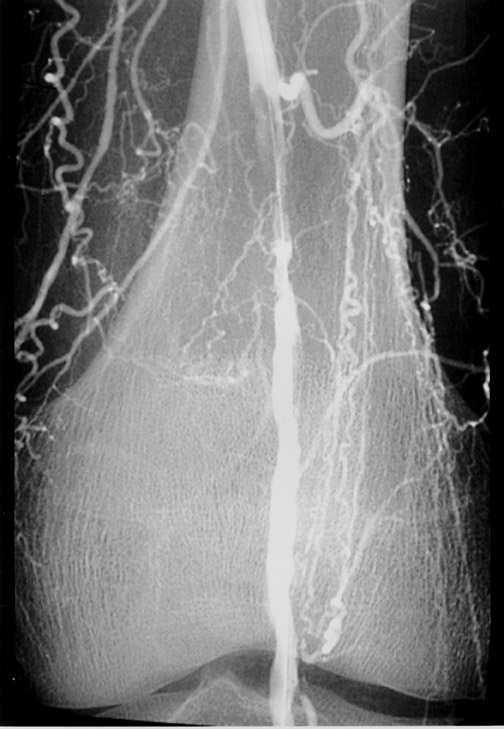
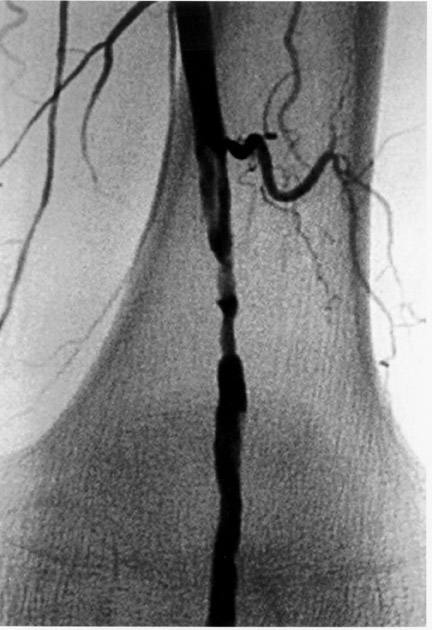
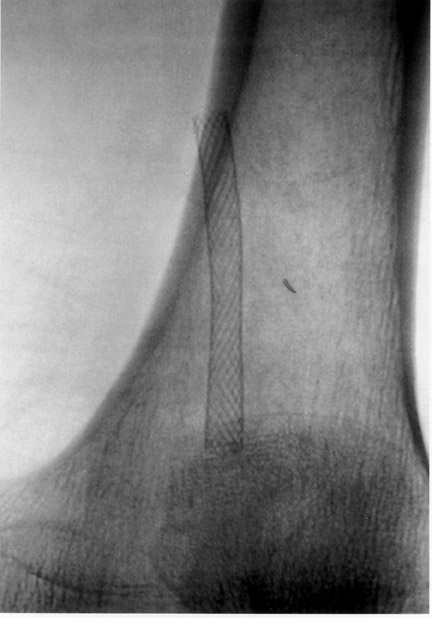
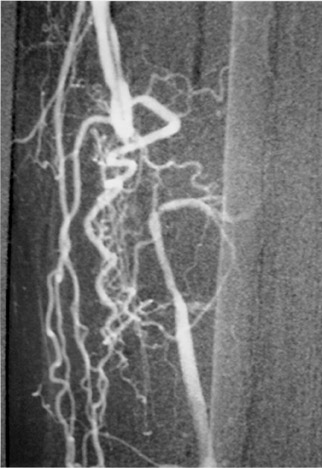
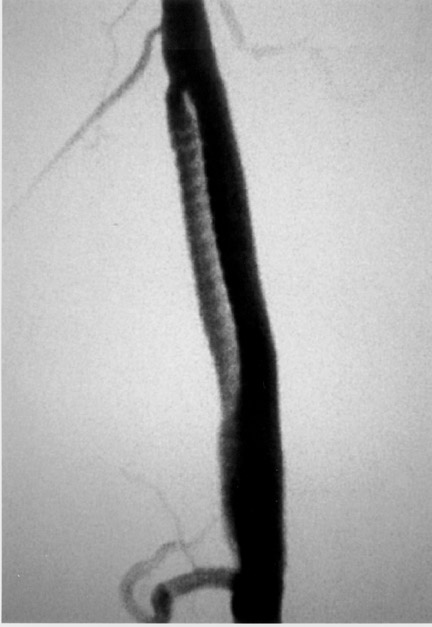
The use of stents below the inguinal ligament is still more limited than in the iliac arteries. If an angiographic unsatisfactory result or a persistent hemodynamically significant residual stenosis remains, stent placement should be considered. Stents are also indicated in cases in which there has been early failure of an angioplasty site (Figures 1 through 4).
Many stents and stent designs are available commercially (Tables 3 through 5). The important features that infiuence stent selection are stent design and material, radiopacity, stent profile, pushabililty, shaft fiexibility, and the familiarity of the operator with the stent design and deployment. The two major classes of stents are the self-expandable (Table 3) and the balloon-expandable stents (Table 4). In certain anatomic locations, such as close to the inguinal ligament, in the adductor canal, and at the knee joint, self-expandable stents provide resistance to permanent stent deformations. Although balloon-expandable stents have a role in selected cases, they are not indicated in arterial segments that are prone to movement due to their inability to resist external compression. Balloon-expandable stents have been crushed by blood pressure cuffs used for segmental limb pressure measurements. Therefore, some operators advocate the sole use of self-expandable stents in the femoropopliteal segment. Stenting below the knee is rarely indicated but, when it is performed, requires a small-vessel stent. A prospective study using self-expandable stents in the femoropop-liteal segment showed that vessel size is important to stent patency. Arteries <5 mm in diameter had a significantly lower long-term patency rate. 7
The stent size should be 10% to 20% larger than the diameter of the vessel to allow satisfactory apposition to the arterial wall. The stent length should match the affected diseased arterial segment, since overstenting might lead to a higher incidence of restenosis. After deployment of self-expanding stents, PTA is necessary to oppose the stent to the arterial wall. Incomplete stent apposition can lead to thrombosis and restenosis. Care should be taken to infiate the balloon inside the stent, since it is well documented that the PTA of the stent edges will lead to restenosis. 8
Many different materials are used in stent designs. There is no superior stent material. The use of a nonferromagnetic stent should be entertained to allow follow-up MRA. All stents cause artifacts on MRA and CTA, but the less metal the better.
Pathophysiology of PTA and stenting
In order to understand the role of PTA and stent placement in the femoral and popliteal arteries, the effect on the arterial physiology must be considered. Percutaneous transluminal angioplasty can lead to plaque rupture, plaque dissection, and overstretching of the vessel wall. The initial luminal gain may be lost by elastic recoil or by dissected plaque tissue. Furthermore, the activation of the coagulation cascade can lead to thrombosis or restenosis. In particular, platelet-derived growth factors cause activation of smooth muscle cells resulting in formation of a neointimal layer, which in its uninhibited form will lead to luminal loss and restenosis. Platelet inhibitors can, in part, block neointimalization.
After stent deployment, the stent's metallic surface is covered with platelets that will, in turn, lead to initiation of the coagulation cascade and deposition of a fibrin layer. The subsequent growth of endothelial cells creates a neointima. This process of re-endothelialization requires 2 to 6 weeks. The implanted stent also induces thrombogenic events, particularly if the stent incompletely apposes the arterial wall. This thrombus formation can prevent the proliferation of endothelial and smooth muscle cells and, ultimately, leads to stent occlusion. Thrombus formation, however, is often self-limiting if the fiow is maintained throughout the stented segment. There is evidence that thrombus formation is more pronounced after stent placement than after PTA alone; however, the initial luminal gain and the reduced constrictive remodeling might more than compensate for the increased intimal hyperplasia. Another factor to consider is the diameter of the femoral and popliteal arteries. Any endoprosthesis will reduce the radius of the vessel and, therefore, infiuence fiow velocity (law of Poiseuille). This factor constitutes the main reason that femoropopliteal prosthetic bypass grafts less then 5 to 6 mm in diameter have a high propensity for occlusion. The overall process of arterial remodeling is still not well understood.
Result of stenting in FPAOD
The most challenging lesions for femoropopliteal intervention are the higher grade lesions (Table 2, type B to type D), which include long-segment stenoses and occlusions. Often, PTA fails due to recoil or intimal dissection. Stents appeared promising in these cases, since they converted early PTA failure into success. The initial enthusiasm was dampened by the high incidence of early thrombosis and restenosis, due to intimal hyperplasia in the stent or at stent edges, converting early gains into late failures 3 to 9 months after intervention. This was confirmed by several prospective studies investigating stent placement in the femoropopliteal segment in a population biased toward claudication rather than rest pain. 7,9-13 The average technical success rate was 98% (range 93% to 100%) with an average complication rate of 7.3% (range 1% to 12%). The primary patency rate, reported as a weighted average, at 1 year was 67% (range 22% to 81%) and at 3 years 58% (range 18% to 72%). These results were reported on an intention-to-treat basis. Gray et al, 13 using balloon- and selfexpandable stents, reported the lowest 1-year primary patency rate of only 22%. The mean length of femoro-popliteal stenosis or occlusion was, however, very long (mean 17 cm). Henry et al, 11 on the other hand, reported the highest 1-year primary patency rate of 81% in 126 patients-again, mainly in patients presenting with claudication. In addition, the average lesion length in this study was only 3.8 cm, again underlining the importance of lesion length for the overall outcome.
Compared with PTA alone, the patency rate for stenting in FPAOD does not appear significantly better. The exception is an increased technical success rate. This is confirmed in Cejna's 14 recent multicenter study, in which 141 patients were randomized to PTA versus Palmaz stent placement in the femoropopliteal segment. The initial technical success rate was significantly higher (99% versus 84%) in the stent group. The cumulative 1- and 2-year angiographic primary patency rates were equal at 63% and 53%, respectively. Also, the secondary 1- and 2-year angiographic patency rates were similar. In their prospectively randomized trial comparing PTA alone with stent placement, Grimm et al 15 found a similar outcome with no significant difference in primary and/or secondary patency rates at 12 and 39 months.
The question of which predictors are associated with early restenosis and stent failure was addressed by Conroy et al, 16 who utilized univariant and multiple logistical regression analysis: better patency rates were associated with larger diameter stents, shorter stents, nonsmokers, and patients who did not require thrombolytic therapy prior to stent placement. Furthermore, patients with claudication had improved stent patency compared with patients with tissue loss. Therefore, stents are not a replacement for PTA in patients with poor runoff or long occlusions.
With the advent of new stent designs, primary stenting in FPAOD has been embraced with fresh enthusiasm. Mewissen 8 reported his experience with a self-expanding nitinol stent in 82 patients with FPAOD. The primary patency rate was 88% at 1 year and 66% at 2 years and was unrelated to the lesion type. Meanwhile, some stent designs have been used in the popliteal artery, which was previously not felt to be a suitable location for stenting because of constant knee bending and relatively small vessel size. Strecker et al 17 reported the use of fiexible tantalum stents in 32 patients. The stents were placed across the patients' knee joints after unsuccessful PTA; they reported 1- and 2-year primary patency rates of 81% and 74%, respectively. Furthermore, preliminary data of a multicenter trial utilizing the Intracoil (ev3, St. Paul, MN) in the femoral arteries are encouraging. In a prospective multicenter study of 37 patients, they reported a primary patency rate of 86% at 12 months and a primary assistant patency rate of 100% at 12 months. 18
The current TASC recommendation 2 regarding stenting in FPAOD is: "a primary approach to the interventional treatment of intermittent claudication and chronic limb ischemia is not indicated. However, stents may have a limited role in salvage of acute PTA failures or complications." The recent studies noted above indicate this may change, and further clinical research into the application of primary stenting for FPAOD is indicated. A recently published decision model 19 comparing PTA, stent placement, and surgical intervention highlights the role of each intervention in the treatment of FPAOD. In utilizing outcome measures (such as lifetime costs, quality adjusted life years, and net health benefit calculations), when compared with surgery and PTA, stents costing ≤$3000 were found to be cost-effective if they could provide a 5-year patency of 70% to 85%.
Result of covered and coated stents in FPAOD
Current research in stent design includes development of drug-coated stents and covered stents. The main reason to use treated stents or covered stents in the femoropopliteal segment is to slow intimal hyperplasia, decrease cellular in-growth, and, ultimately, prevent restenosis and occlusion. Covered stents improve initial technical success by treating transections and dissection fiaps and by stabilizing plaque material. The impact of graft material, the position of the graft (on the inside or outside of the stent), the pore size, and the thickness of the graft optimal for the arterial system are still under investigation. It appears that re-endothelialization of the stent graft is fairly slow in humans. Further experimental animal data suggest an increased degree of neointima formation and infiammatory vessel wall reaction compared with noncovered stents, depending on the graft material used.
Initial experience by Henry et al 20 utilizing a polyester-covered stent showed a significant incidence of fever and local pain in 26 patients after implantation. Similar clinical observations were made by Ahmadi et al, 21 who found a significant postimplantation syndrome with fever and C-reactive protein (CRP) elevation in 40% of the patients utilizing a Dacron-covered prosthesis. More than half of the patients also reported persistent pain at the implantation site for >5 days. The primary patency rates were also somewhat disappointing, with only 23% at 1 year and 17% at 3 years, respectively. Henry et al, 22 on the other hand, reported on the use of 119 covered stents in the femoropopliteal segment to treat a variety of aneurysmal and occlusive lesions with great success. The primary and secondary patency rates at 27 months were 64% and 76%, respectively.
This enthusiasm, however, gave way to some disappointing realism. A prospective multicenter trial, the Hemobahn multicenter trial, 23 using a fiexible nitinol stent covered with thin-walled radially enforced ePTFE, was slightly discouraging. The initial report included stenting of iliac and femoropopliteal arteries in 141 limbs; 90% of all patients were claudicators. Eighty femoral lesions were treated; 72% were severity type A or B. Although the technical success rate was 100%, the initial acute thrombosis rate was 4% and the reocclusion rate at 1 year was 20%. The primary patency rate was 90% at 6 months and 79% at 12 months, respectively. The mean lesion length was 13 cm in this study, a length that would likely be associated with very poor outcome with PTA alone.
To add to the confusion, Deutschmann, 24 in a single-center study, reported disappointing results using the same stent-graft. The technical success rate was 94%; the primary patency rate at 3 and 6 months was only 61% and 49%, respectively. Twenty-two percent of all patients had early reocclusions at <1 month, and an additional 49% of all grafts were occluded at an average of
7.6 months. Significant intimal hyperplasia was seen at the leading and trailing edges of the stent, and the highest reocclusion rate was seen in stents >10 cm in length. Interestingly, a recent single-center, randomized comparison of PTA and placement of the same Hemobahn covered stent (Gore Inc., Tempe, AZ) came to an opposite conclusion. Patients treated with the stent-graft had significantly superior 2-year primary patency rates: 87% versus 25% for the PTA group. In addition, improved clinical outcome was shown for the stent-graft group with a variety of parameters. 25
Therefore, it appears that covered stents have potential, particularly in complex femoropopliteal lesions. However, further prospective randomized studies are necessary to interpret the role of covered stents in the treatment of FPAOD. Today, three covered stent designs are available; they differ in stent material and covering graft used (Table 5).
More recently, coated stents have been used for the treatment of FPAOD. The initial 6-month results of a double-blind, randomized, prospective multicenter trial included 36 patients with FPAOD. These patients, who had an average lesion length of 8.5 cm, were randomized to be treated with a sirolimus-eluting nitinol stent versus an uncoated nitinol stent. At 6 months, the in-stent restenosis was 22% in the sirolimus-elut-ing stent group versus 31% in the uncoated stent group. A significant larger in-stent mean luminal diameter was found in the coated stent group. 26 These promising early results might indicate a true quantum leap in stent technology for FPAOD intervention.
Conclusion
Percutaneous intervention for FPAOD is a well-established treatment option for low-grade lesions that results in high technical success rates and good long-term patency rates. For short lesions, PTA remains the treatment of choice. If PTA fails, secondary stent placement is indicated, which has been found to have patency rates similar to those of PTA. Complex lesions seem to benefit from primary stent placement, potentially leading to improved greater patency rates and clinical advantage. The use of covered stents must still be determined. The initial results with coated stents are promising; however, long-term studies are necessary to identify the subgroup of patients who will benefit from this device.