Diagnostic imaging of spinal fusion and complications
Images
Dr. Hayeri is a former Research Fellow, Division of MSK Imaging at University of California, Irvine Medical Center, Orange, CA, and Dr. Tehranzadeh is Chief of Radiology at Long Beach VA and Professor Emeritus and Vice Chair of Radiology, Department of Radiological Sciences, University of California, Irvine Medical Center, Orange, CA.
Back pain is the most common cause of limited activity in people younger than 45 years in the United States. It is the second most frequent reason for visits to a physician and ranks fifth as the reason for hospital admission.1 It is estimated that 18% of the U.S. population experience low-back pain each year. Fortunately, in most cases, the underlying pathology is benign and the pain is self-limited. Noninvasive methods of treatment such as physical therapy and pharmacotherapy typically resolve such pain.
Treatment of back pain is the third most common indication for surgical procedures in the nation. Decompression and occasional arthrodesis with frequent instrumentation are the main surgical procedures performed in the U.S.2 It is a common belief that immobilization and/or removal of the painful segment decreases pain. Failed back surgery syndrome (FBSS) is defined as failure to relieve lower back pain symptoms following surgery. In the best of all situations, this syndrome occurs after a minimum of 20% of spine fusion surgeries. The syndrome can result from: mistaken diagnoses, technique error, poor application, inappropriate indication, pseudarthrosis or continued natural progression of disease. This syndrome can be prevented to a large extent by meticulous pre- and intraoperative radiologic examination.3,4 Since the initial description of spinal instrumentation by Harda in 1889 and subsequent spinal fusion surgery by Fred Albee and Russel Hibbs in treatment of spinal tuberculosis in 19115–7 there have been a great many advances in surgical methods and instrumentation, as well as many more indications for fusion. Among current indications are scoliosis, spondylolisthesis, congenital deformities, spinal instability in trauma or by iatrogenic causes (e.g. extensive laminectomy), infection and neoplasm. The current indication for spinal arthrodesis is broad and it includes the category of degenerative disc diseases.3
Postoperative imaging is used to assess disease progression, positioning of instrumentation, possible complications and the extent of bone-graft fusion. Knowledge of the advantages and limitations of different imaging modalities is necessary for optimal evaluation of patients with spinal instrumentation. Radiologists should also be familiar with different surgical methods used in spinal fusion, types of instrumentation and potential complications to properly appraise postoperative images.
Stability is described as resistance of the spine to deformation under physiologic stress. Mulholland8 in a recent review of instability and low-back pain hypothesized that the cause of low-back pain could be due to abnormal disc loading. Currently, the most widely accepted cause of low-back pain and the underlying concept promoting the use of spinal fusion is nonphysiologic movement of the degenerated segment. Most appliances are placed to provide stability during bone fusion, and their function is complete when this has occurred. Be-cause of the morbidity associated with repeated surgery, intact implants are generally left in place for life. Fractured and dislodged implants are often removed because of the need for revision and the potential for migration of the components, leading to substantial soft-tissue or neural injury.
Spinal instrumentation
Surgical implants in spinal surgeries are used to stabilize the spine, replace the defective parts and maintain anatomic reduction. Internal spinal instrumentation has undergone considerable advances during the last century. Radiologists should be able to identify the devices most commonly used and understand their biomedical principles and specifications.
Common devices
Rods, plates and rectangles
Rods can extend to single or multiple spine segments. They can be single or double, straight, L-shaped or can be cut and fashioned as required. They are attached to the spine by hooks, pedicle screws or sublaminar or interspinous wires or cables. Rods are usually preferred over plates for multisegment fusion because of their ability to span a long segment. The Hartshill rectangle is seldom used today. It is a stainless-steel rectangle that attaches to the spine by sublaminar wires and occasionally interspinous wires. Various shapes of plates in different sizes have been developed for anterior or posterior spine fusion.9–11 Some of the commonly used instruments and systems, and their specifications, are summarized in Table 1.
Translaminar or facet screws
These devices can be used when posterior spinal elements are intact. They attach the lamina of 2 adjacent vertebrae.
Interbody spacers
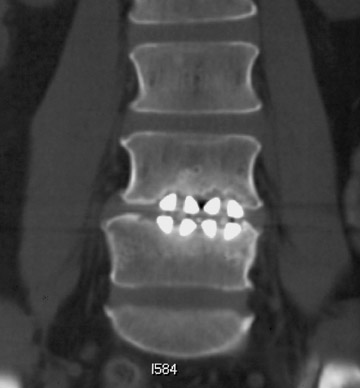
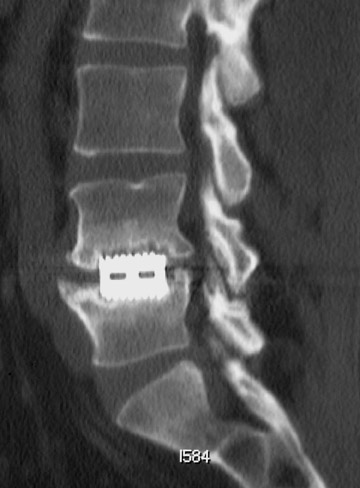
Interbody spacers could be solid (ramp) or hollow (cages). Cages are filled with bone-graft material and inserted into the intervertebral space or replace a vertebra after its removal (i.e. corpectomy). Cages are usually made of titanium carbon fibers, polyetherether ketan (PEEK) or of cortical bone graft. Most cages contain 2 radiopaque markers to identify their position in radiographs and to enable their assessment. They are made in different shapes based on the method of approach to the intervertebral disc.
In anterior interbody fusion (AIF), cages are more round in shape, while in posterior interbody fusion (PIF) they are more rectangular. Transforaminal interbody fusion (TIF) cages are more crescent-shaped. Expandable cylindrical or mesh cages are used in vertebral body replacement procedures.
Cages are usually supported by additional posterior, anterior or lateral instrumentation (i.e. screw with plates or rods) to increase stability. For a standalone interbody fusion cage, the interbody spacer is fixed to the adjacent vertebral body with screws to eliminate the need for additional instrumentation support. Retropulsion of the cage is a possible complication, but is more common in PIF.12 A distance of ≤2 mm between the cage’s posterior marker and the posterior margin of the vertebra should exist to provide reassurance that the cage is not invading the spinal canal.11 Cage subsidence (defined as migration of >3 mm into the adjacent vertebra) and lateral displacement is a disadvantage of using mesh and standalone cages.13–15 The incidence of subsidence is reported from 18% to ≤62.5% in patients who undergo spinal procedures with standalone cervical cages. Expandable cages have broader surface area and duller edges at both ends, which minimize their subsidence and also allow immediate load bearing and stability after corpectomy.16
Miscellaneous
Dynamic stabilization devices are a new category of instruments that are in various stages of development. They can be used alone or in conjunction with other instrumentation. They act by controlling the abnormal motion and uneven load in segments adjacent to the level of fusion in order to minimize progressive degeneration. Artificial ligaments (e.g. Dynamic Stabilization System [Dynesys], Zimmer Inc., Warsaw, IN), interspinous decompression systems (e.g. X-STOP Spacer, Medtronic Spine, Memphis, TN; and the Wallis Dynamic Posterior Stabilization System, Zimmer Inc., Bordeaux, France), and posterior element replacement systems (e.g. Total Facet Arthroplasty System, Archus Orthopedics, Redmond, WA) are examples of such devices.11
Surgical methods
Surgical techniques can be divided on the basis of perceived patient morbidity into minimally invasive or traditional-open procedures performed via either an anterior or posterior approach. In interbody fusion, the intervertebral disc or a complete vertebra is removed and replaced with bone graft. Interbody fusion of the spine can be approached anteriorly or posteriorly.
Anterior interbody fusion (AIF) has the advantage of a broader access to the disc space. However, it is limited by potential injury to major vessels and sympathetic nerve chain.17 Oskouian and Johnson reported a 5.8% incidence (12 of 207 patients) of vascular complications in patients who underwent anterior thoracolumbar spine reconstruction procedures.18
Extreme lateral interbody fusion (XLIF) is a newer surgical approach to fuse L1 to L5 and to minimize disadvantages of AIF. Extreme lateral interbody fusion approaches the anterior spine from the flank.
In posterior interbody fusion (PIF) bilateral laminectomies are performed and bone-graft material is inserted into the disc space after the disc is removed. Posterior interbody fusion has the disadvantage of potential injury to nerve roots. Retrograde migration of the graft or cage is also more common with the posterior approach.19
Transforminal interbody fusion (TIF) is a modified PIF that uses a more lateral approach and thus leaves the midline bone structures intact. Min et al. showed both AIF and PIF can produce good outcomes in treating lumbar spondylolisthesis, but AIF is more advantageous in preventing the development of adjacent segment degeneration.20
Overall, Lemcke et al. reported that, with regard to the indications and contraindications, AIF and PIF are unquestionably accepted as up-to-date methods.21 The decision to use AIF or PIF is mainly based on the patient’s presenting pathology, spine anatomy, the surgeon’s experience, history of previous surgery and other conditions that may favor one approach over another (e.g. AIF is difficult in the presence of marked vascular calcification).11,22 Laparoscopic interbody fusion can also be performed; however, compared with open surgery, the overall complication rate is higher (19% vs. 14%, respectively).
Posterolateral fusion is an alternative for interbody fusion. In posterolateral fusion, adjacent vertebrae are fused together by placing the bone-graft material between the transverse processes. In comparison, interbody fusion provides a greater surface area of bone contact and produces a more favorable fusion compared to the posterolateral method.23 Addition of instrumentation to interbody fusion increases success rates to nearly 100%. Using cages in interbody fusions provides more immediate stability during bone graft incorporation.23–25
Imaging of postoperative spine fusion
Postoperative imaging plays an important role in the assessment of fusion and bone formation. It is also helpful to detect instrument failure and other suspected complications. It is necessary to compare current images with previous studies to identify any subtle changes and disease progression.
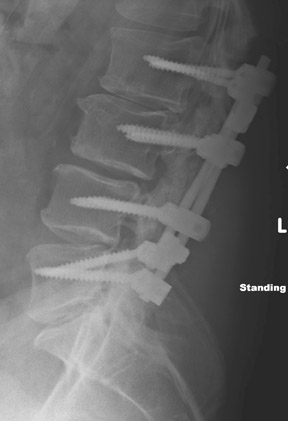
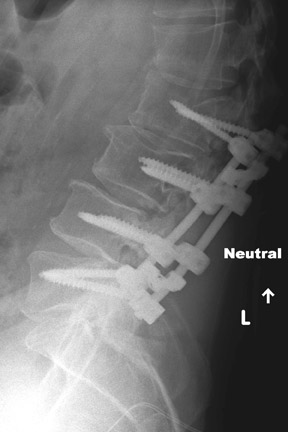
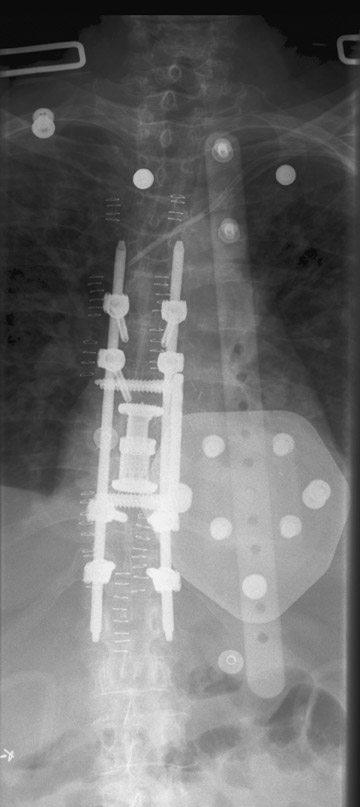
Evaluation of the postoperative spine usually begins with conventional radiographs in AP and lateral projections. It usually takes 6 to 9 months for a solid bone fusion to be established radiographically. Conventional radiographs are capable of detecting instrument failure, infection and other causes of failed fusion (Figures 1 through 7). Additional views in lateral flexion and extension are sometimes used to evaluate the presence of motion and the integrity of the fusion.17 Ray defined 6 criteria to radiographically verify a solid fusion:
- no motion or <3 degrees of intersegment position change on lateral flexion and extension views,
- lack of a lucent area around the implant,
- minimal loss of disc height,
- no fracture of the instrument, bone graft or vertebrae,
- no sclerotic change in the graft or adjacent vertebrae, and
- visible osseous formation in or around the cage.26
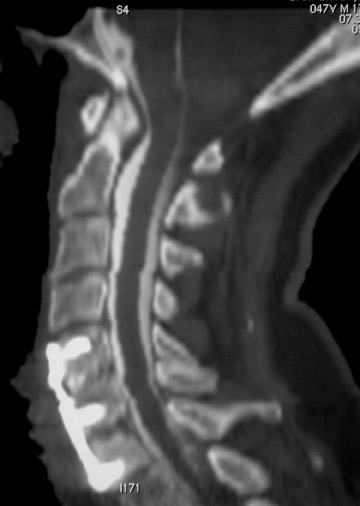
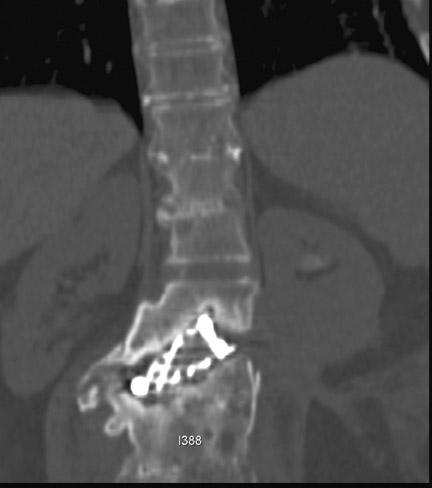
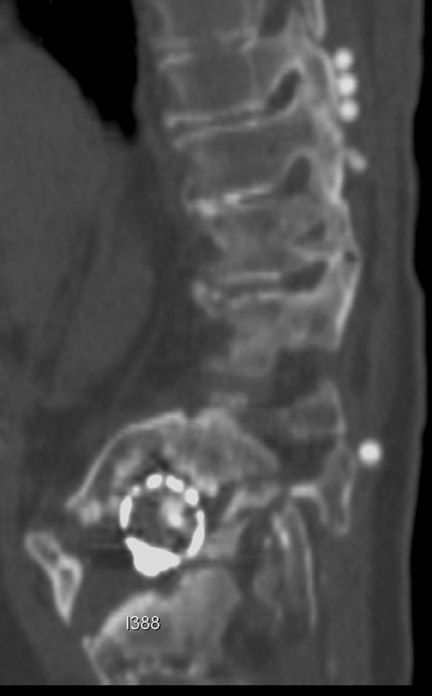
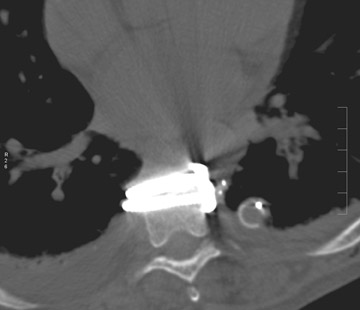
Sometimes radiographs are nondiagnostic and, based on clinical suspicion and the type of the applied instrument, additional imaging with other modalities may be applied. Currently, computed tomography (CT) with multiplanar reconstruction (MPR) is considered the modality of choice for imaging bony detail and assessing osseous formation and hardware position despite artifact formation. CT is also useful in demonstrating the spinal canal and its alignment and is capable of detecting infection and pseudarthrosis12 (Figure 8). Cook et al. evaluated the extent of bony fusion in an animal model and reported that CT was capable of detecting fusion in 83% of cases, but coincidence of CT image results with histological findings was present in only 14% of specimens and CT significantly overestimated the extent of fusion.27
In another study, Heithoff et al. compared CT images with reoperation findings in symptomatic pseudarthrosis patients and reported that CT was not reliable in identifying these patients.28 Artifacts are the primary disadvantage of CT although artifacts are seen less commonly with titanium implants compared with stainless steel because of the lower beam attenuation coefficient of titanium implants.11
Magnetic resonance imaging (MRI) has been used increasingly in recent years since introduction of titanium-based implants with reduced artifact compared to formerly used stainless-steel devices. These artifacts could be decreased even more by changing imaging parameters such as reducing echo time, increasing bandwidth and decreasing voxel size. Aligning the implant along the axis of the magnetic field also reduces artifact although it is often not completely achievable due to the multidirectional configuration of most hardware. Spin echo sequences are less vulnerable to magnetic susceptibility artifact and give better quality images compared with gradient echo sequences. MRI is useful in detecting infection (Figure 9) and assessing recurrent tumor. MRI is the modality of choice in assessing intraspinal contents. Myelography (Figure 6) is an alternative when MRI is contraindicated or is nondiagnostic because of artifact.
Radionuclide scans are mainly used to detect infection.29 Early stages of pseudarthrosis can also be assessed by increased radionuclide uptake, although this may appear indistinguishable from remodeling. Sonography is used to detect fluid collections and abscesses in the postoperative spinal fusion.17
Spinal fusion instrumentation and complications
Potential complications of spinal surgery vary based on the site of surgery, surgical approach, underlying disease, applied instrumentation, surgeon skill and other clinical factors. Besides the common complications associated with spinal fusion procedures; there are some additional complications based on site, procedure and type of instrumentation.
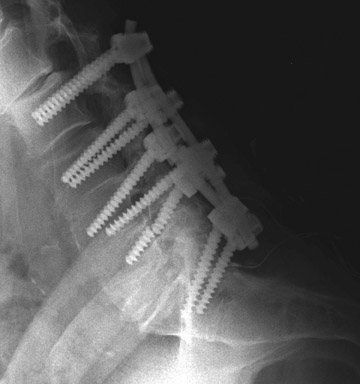
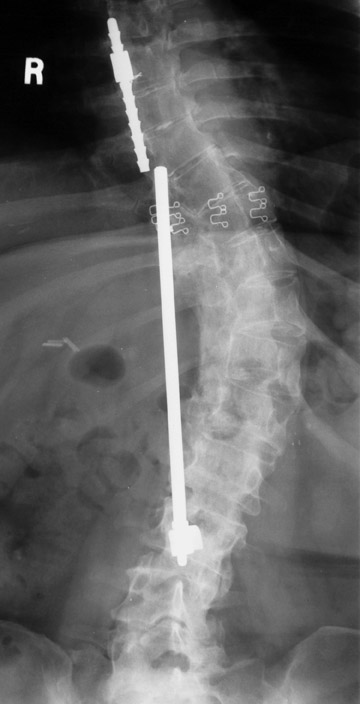
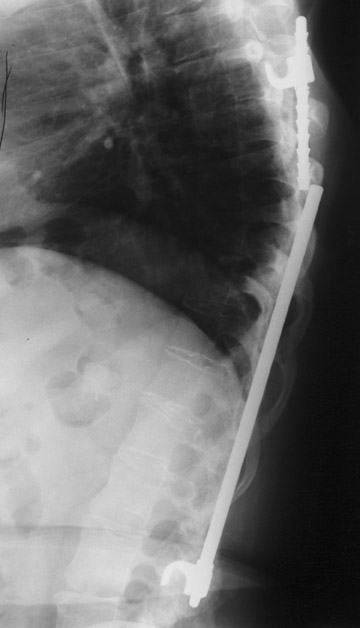
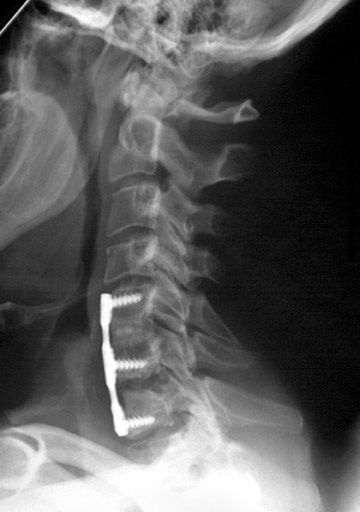
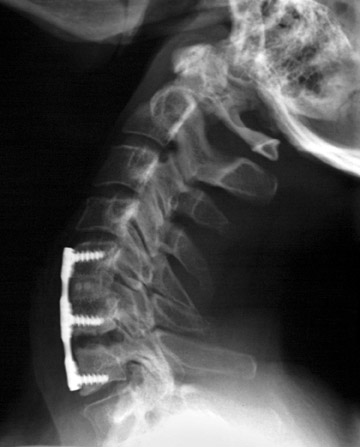
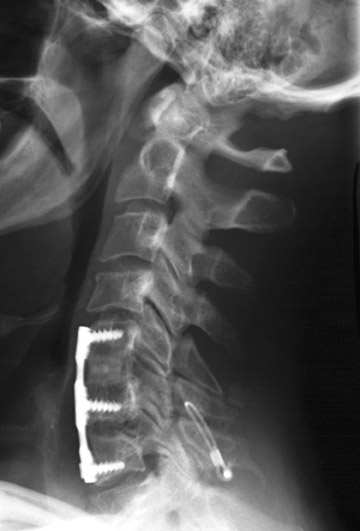
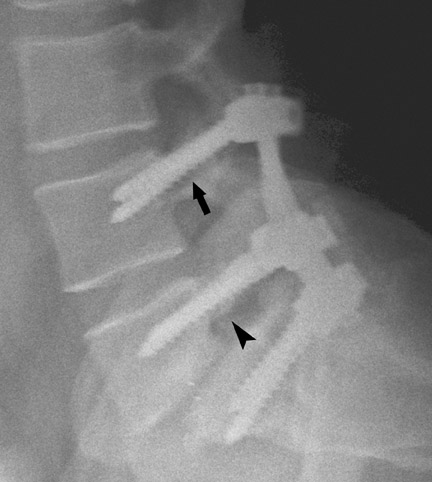
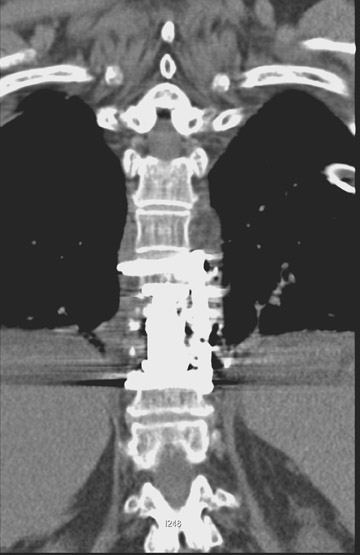
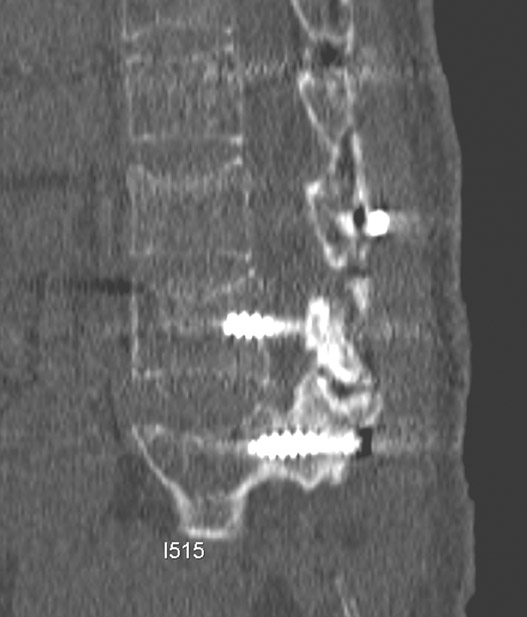
Hardware fracture (Figures 1 through 4) occurs most commonly as a result of metal fatigue from the repeated stress in spinal movements. The fractured appliance may not be displaced, making its detection difficult. A dislodged or fractured appliance does not necessarily indicate instability or clinical failure of the fusion but is most frequently associated with motion, instability and pseudarthrosis.30 The prominence of the instruments can cause chronic tissue irritation leading to pain, bursa formation and even pressure sores with tissue necrosis. This is an occasional indication for hardware removal.30 There is also a risk of bone resorption around screws or under the implants that are in direct contact with the bone (Figures 5 and 7). This will cause the bones to weaken and predisposes them to fracture and it leads to hardware failure. A loose appliance repeatedly moves and produces bone resorption or erosion. Fused bones are less mobile, which makes the bones vulnerable to fractures above or below the implants if subjected to trauma (Figure 10). Unsuccessful fusion may have other causes such as development of facet arthritis (Figure 6C) or disc disease above or below the fusion level.3 Premature degenerative changes at the disc levels above and below the fused segment can occur due to the reduced number of mobile segments. This complication is reported in 10.2% of patients with posterior fusion and instrumentation.31
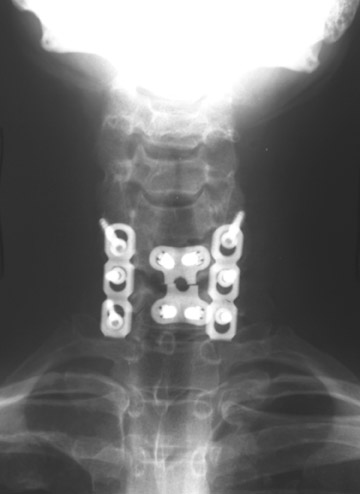
In the cervical spine, potential complications of the posterior approach are mainly neurological and include dural, nerve root or cord injury. The anterior approach is associated with risks of injuring the main vascular structures (carotid and vertebral arteries, jugular vein), causing recurrent damage to the laryngeal nerve or soft tissue, such as the esophagus, trachea or lungs (Figure 11). Postoperative complications include hematoma, pseudomeningocele, infection and instability as a result of laminectomy or incorrect hardware placement. Wires and cables are used as a primary or supplementary instrument in stabilizing the posterior cervical spine (Figure 6). Complications include breakage and slippage of skeletal attachments. Cables (e.g. Songer cable) are much more resistant to fatigue fracture and failure. Plates are used for the anterior and posterior cervical spine. They are also prone to fracture and failure (Figure 2). Screws may break or dislodge or may be misplaced and impinge the cord or nerve root when placed posteriorly.17 In a retrospective study of 1015 patients who underwent anterior cervical discectomy for cervical radiculopathy and/or myelopathy due to degenerative disc disease and/ or cervical spondylosis, Fountas et al. reported the most common postoperative complications to be dysphagia (9.5%), postoperative hematoma (5.6%) and recurrent laryngeal nerve palsy (3.1%).32
Screws should approach the opposite cortex but should not breach it. In anterior-plate screw fixation, the screws may back out and impinge soft tissue (e.g. great vessels, trachea and esophagus) or overpenetrate the posterior cortex and impinge on the cord. These complications can be prevented by using a cervical-spine locking plate with screw caps (e.g. Morscher). This device prevents the screws from backing out and provides increased holding power removing the need for transcortical purchase with the risk of over penetration.
Immobility of the fused segment causes additional stress on adjacent levels of the vertebral column. Ossification of anterior longitudinal ligament and facet disease are common complications of anterior plate and screw fixation (Figure 6).9,17
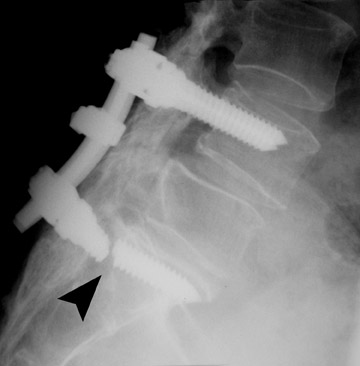
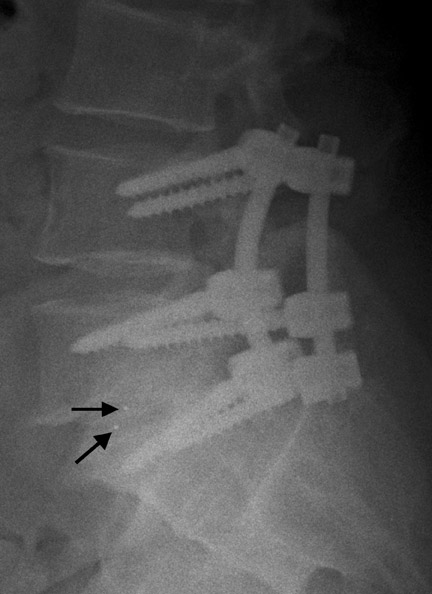
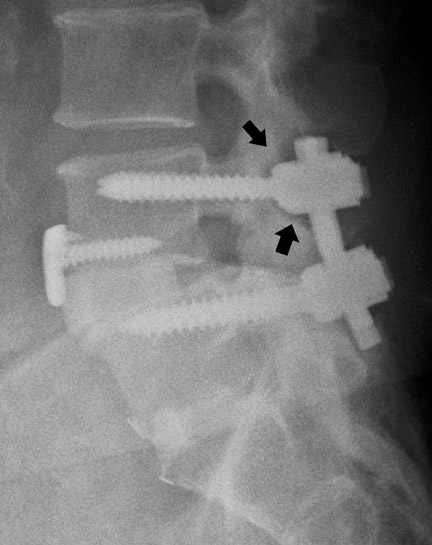
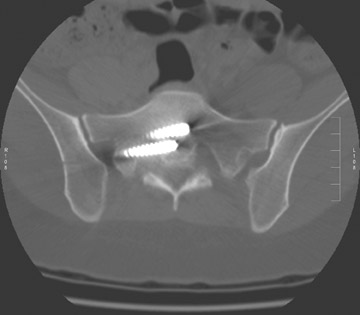
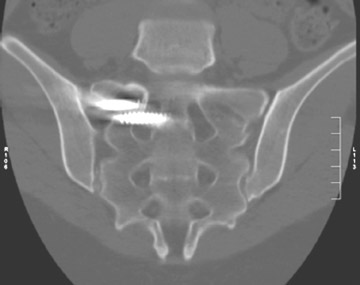
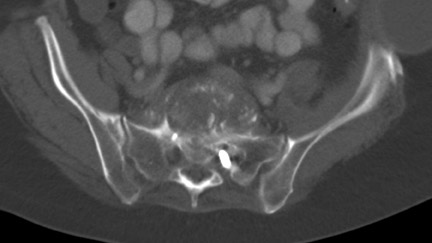
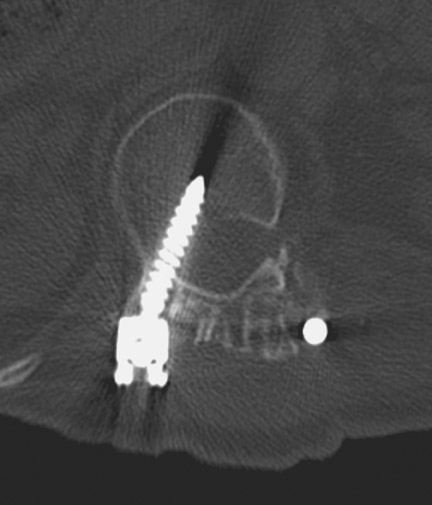
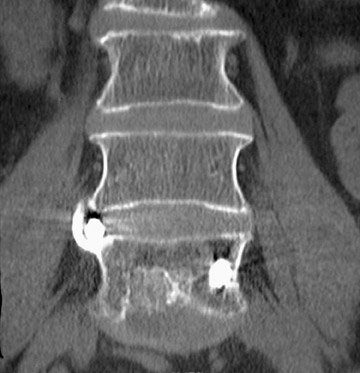
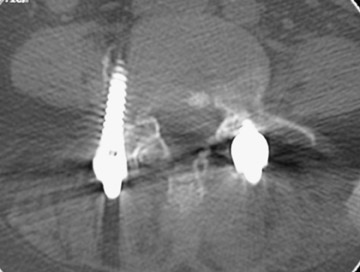
In anterior fusion of the thoracic or lumbosacral spine, the devices should be laterally located in the anterior column. Neurologic deterioration is the most-feared complication of surgery and may be caused by hardware movement or malpositioned screws (Figures 12 through 15). Incorrect use and later dislodgment or fracture of instruments may also contribute to complications such as instability, fusion failure or pain—with possible resultant neurologic damage. Postoperative neurologic complication due to lumbar instrumentation has been reported in 3% to 11% of patients undergoing spinal procedures. Postoperative neurologic injuries can also be due to cord edema or hematoma and are often self-limited.30 Bone graft material can migrate or hypertrophy resulting in impingement on the spinal canal or neural foramen.17,33,34 Radiographs often show the failed instrument that may have caused neurologic deterioration. Rare but life-threatening complications such as delayed aortic rupture due to instrumentation have also been reported.35
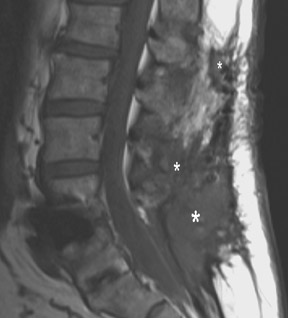
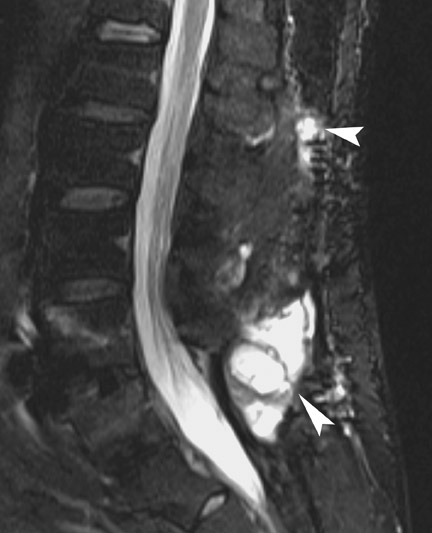
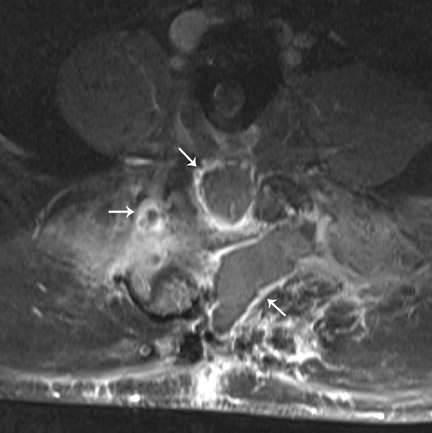
Infection is reported in 1% to 2.4% of patients undergoing lumbar instrumentation. Infection leads to bone destruction and resorption around the implant. On imaging, a lucent area around an implant implies a loose appliance and potential infection (Figures 8 through 9). CT-guided aspiration can be used to isolate the microorganism. Unlike superficial infections that can even be diagnosed clinically, deeper infections such as discitis are sometimes more challenging. Osteomyelitis in adjacent vertebrae, disc collapse and destruction indicate discitis radiographically. Radionuclide-labeled white blood cell scintigraphy and MRI can be helpful to detect infection in early stages.36
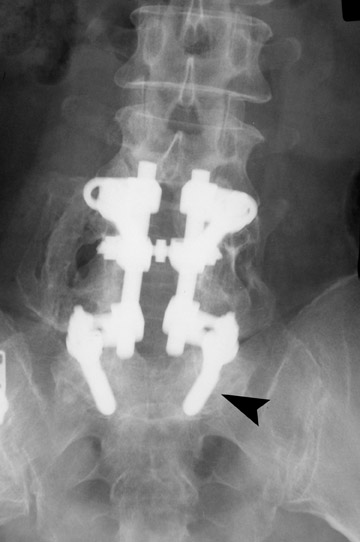
Failed fusion with the development of pseudarthrosis is a common end result of implant failure or improper surgical technique (Figures 5 and 6). Its incidence in lumbar instrumentation is reported in 5% to 32% of patients. CT is the optimal method for evaluating a bone graft. A failed fusion with pseudarthrosis formation results in continued stress on the implant, and hardware fracture is inevitable. Suda et al. described radiological risk factors for pseudarthrosis and/or instrument breakage after PLF with pedicle screws to be related to preserved disc height and the presence of segmental kyphosis.37
The risk of pseudarthrosis escalates with increased patient age and smoking. Pseudarthrosis is more common using external braces than internal fixation. The rate of pseudarthrosis is decreased with meticulous surgical technique, including careful facet excision and adequate graft placement. Repair is necessary if the patient presents with implant failure or pain. In asymptomatic patients, intervention may be deferred and the patient’s condition should be followed.38,39
Conclusion
Radiologists face new challenges as the number of, and indications for, spinal surgery grow. Adequate understanding of various surgical techniques and instruments, coupled with improved awareness of the possible complications, are vital when interpreting postoperative studies. Radiologists should carefully compare these critical points with baseline studies to develop a targeted assessment of grafts and hardware. With more familiarity of postoperative spinal images obtained on various modalities and the knowledge of how certain situations (e.g. surgical technique and hardware) contribute to failed back surgery syndrome, radiologists can quickly arrive at a precise diagnosis, permitting appropriate treatment and minimizing patient suffering.
REFERENCES
- Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581-585.
- Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine. 2005;30:1441-1445; discussion 1446-1447.
- Tehranzadeh J, Ton JD, Rosen CD. Advances in spinal fusion. Semin Ultrasound CT MR. 2005;26:103-113.
- Rosales-Olivares LM, Miramontes-Martínez V,Alpízar-Aguirre A, et al. Failed back surgery syndrome. Cir Cir. 2007;75:37-41.
- Hadra BE. The classic: Wiring of the vertebrae as a means of immobilization in fracture and Pott’s disease. Berthold E. Hadra. Med Times and Register. 1891;Vol 22. Clin Orthop Relat Res. 1975; 112:4-8.
- Albee FH. The classic: Transplantation of a portion of the tibia into spine for Pott’s disease. JAMA. 1911;57:885-887.
- Hibbs RA: A report of 59 cases of scoliosis treated by fusion operation. By Russel A. Hibbs, 1924. Clin Orthop Relat Res. 1988;229:4-19.
- Mulholland RC. The myth of lumbar instability: The importance of abnormal loading as a cause of low-back pain. Eur Spine J. 2008;17:619-625.
- Slone RM, MacMillan M, Montgomery WJ. Spinal fixation. Part 1. Principles, basic hardware, and fixation techniques for the cervical spine. Radiographics. 1993;13:341-356.
- Slone RM, MacMillan M, Montgomery WJ, Heare M. Spinal fixation. Part 2. Fixation techniques and hardware for the thoracic and lumbosacral spine. Radiographics. 1993;13:521-543.
- Rutherford EE, Tarplett LJ, Davies EM, et al. Lumbar spine fusion and stabilizaion: Hardware, techniques, and imaging appearances. Radiographics. 2007;27:1737-1749.
- Berquist TH. Imaging of the postoperative spine. Radiol Clin North Am. 2006;44:407-418.
- Bartels RH, Donk RD, Feuth T. Subsidence of standalone cervical carbon fiber cages. Neurosurgery. 2006;58:502–508.
- Gercek E, Arlet V, Delisle J, Marchesi D. Subsidence of stand-alone cervical cages in anterior interbody fusion: Warning. Eur Spine J. 2003; 12:513–516.
- Barsa P, Suchomel P. Factors affecting sagittal malalignment due to cage subsidence in standalone cage assisted anterior cervical fusion. Eur Spine J. 2007;16:1395-1400.
- Riaz S, Fox R, Lavoie MV, Mahood JK. Vertebral body reconstruction for thoracolumbar spinal metastasis – a review of techniques. J Ayub Med Coll Abbottabad. 2006;18:70-77.
- Slone RM, MacMillan M, Montgomery WJ. Spinal fixation. Part 3. Complications of spinal instrumentation. Radiographics. 1993;13:797-816.
- Oskouian RJ Jr, Johnson JP. Vascular complications in anterior thoracolumbar spinal reconstruction.J Neurosurg. 2002;96(1 Suppl):1-5.
- McAfee PC. Interbody fusion cages in reconstructive operation on the spine. J Bone Joint Surg [AM].1999;81-A:859-878.
- Min JH, Jang JS, Lee SH. Comparison of anterior- and posterior-approach instrumented lumbar interbody fusion for spondylolisthesis. J Neurosurg Spine. 2007;7:21-26.
- Lemcke J, Klötzer S, Klötzer R, Meier U. PLIF and ALIF for the degenerative spondylolisthesis of the lumbar spine. Z Orthop Ihre Grenzgeb. 2007; 145:48-54.
- Blumenthal SL, Ohnmeiss DD; NASS. Intervertebral cages for degenerative spinal diseases. Spine J. 2003; 3:301-309.
- Ekman P, Moller H, Tullberg T, et al. Posterior lumbar interbody fusion versus posterolateral fusion in adult isthmic spondylolisthesis. Spine. 2007;32: 2178-2183.
- Weatherley CR, Prickett CF, O'Brien JP. Discogenic pain persisting despite solid posterior fusion. J Bone Joint Surg Br. 1986;68:142-314.
- Brantigan JW, Steffee AD, Lewis ML, et al. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: Two-year results from a Food and Drug Administration investigational device exemption. Clinical trial. Spine. 2000;25:1437-1446.
- Ray CD. Threaded fusion cages for lumbar inter-body fusions: An economic comparison with 360 degrees fusions. Spine. 1997;22:681–685.
- Cook SD, Patron LP, Christakis PM, et al. Comparison of methods for determining the presence of anterior lumbar interbody fusion. Spine. 2004;29:1118-1123
- Heithoff KB, Mullin WJ, Holte D, et al. The failure of radiographic detection of pseudoarthrosis in patients with titanium lumbar interbody fusion cages. Paper presented at: International Society for the Study of the Lumbar Spine; June 1999; Kona, HI.
- Berquist TH, Currier BL, Broderick DF. The spine. In: Berquist TH, editor.Imaging atlas of orthopedic appliances and prosthesis. New York, NY:Raven Press;1995:109-215
- Heller JG, Whitecloud TS III, Butler JC, et al. Complications of spinal surgery. In: Rothman RR, Simeone FA, eds. The spine. 3rd ed. Philadelphia, PA:Saunders;1992:1817-1898.
- Cho KJ, Suk SI, Park SR, et al. Complications in posterior fusion and instrumentation for degenerative lumbar scoliosis. Spine. 2007;32: 2232-2237.
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine. 2007;32: 2310-2317.
- Lowery GL, McDonough RF. The significance of hardware failure in anterior cervical plate fixation. Patients with 2- to 7-year follow-up. Spine. 1998;23:181-186; discussion 186-187.
- Spanu G, Marchionni M, Adinolfi D, Knerich R. Complications following anterior cervical spine surgery for disc diseases: An analysis of ten years experience. Chir Organi Mov. 2005;90:229-240.
- Ohnishi T,Neo M, Matsushita M, et al. Delayed aortic rupture caused by an implanted anterior spinal device. Case report. J Neurosurg. 2001;95 (2 Suppl):253-256.
- Young PM, Berquist TH, Bancroft LW, Peterson JJ. Complications of spinal instrumentation. Radiographics. 2007;27:775-789.
- Suda K, Ito M, Abumi K, et al. Radiological risk factors of pseudoarthrosis and/or instrument breakage after PLF with the pedicle screw system in isthmic spondylolisthesis. J Spinal Disord Tech. 2006;19:541-546.
- Schlegel J, Yunan HA, Fredricksen B. Anterior interbody fixation devices. In: Frymoyer JW, Ducker TB, eds. The adult spine: principles of practice. New York, NY:Raven;1991:1947-1959.
- Emami A, Deviren V, Berven S, et al. Outcome and complications of long fusions to the sacrum in adult spine deformity: Luque-galveston, combined iliac and sacralscrews, and sacral fixation. Spine. 2002;27:776-786.
Related Articles
Citation
. Diagnostic imaging of spinal fusion and complications. Appl Radiol.
August 6, 2009