Considerations in the Selection of a New Gadolinium-Based Contrast Agent








CE Accreditation Information
Date of Release: 1/15/14
Date of Expiration: 1/31/16
Estimated time to complete: 1 Hour
Target Audience
Radiologic Technologists
Learning Objectives
Upon completion, participants should:
• Understand the physicochemical similarities and differences among the available GBCAs
• Understand how the chemical design of each agent affects its relaxivity
• Understand the important practical issues of stability and safety
To claim credits, visit www.appliedradiology.org/cc5
Dr. Tweedle is a Stefanie Spielman Professor of Radiology at The Ohio State University, Columbus, OH; Dr. Kanal is Professor of Radiology and Neuroradiology at the University of Pittsburgh Medical Center, Pittsburgh, PA; and Dr. Muller is in the Department of General, Organic & Biochemical Chemistry, University of Mons, Mons, Belgium.
The use of gadolinium-based contrast agents (GBCAs) to enhance the sensitivity and specificity of magnetic resonance imaging (MRI) has been part of standard clinical practice for over 2 decades. Currently, there are 9 GBCAs approved by the U.S. Food and Drug Administration (FDA) that vary in a number of properties, some of which may significantly impact their clinical utility, particularly for specific applications. So what criteria should radiologists use to select a GBCA? Of the various physicochemical properties, the criteria most important to radiologists for optimization of diagnostic efficacy and patient safety are relaxivity and stability.
A GBCA with a higher relaxivity provides increased signal intensity, greater contrast enhancement, and improved diagnostic efficacy.1-3 In addition, the higher signal seen with higher-relaxivity agents affords the potential to use lower doses in patients at risk of developing nephrogenic systemic fibrosis (NSF).4-9 Stability is an important consideration because free gadolinium (Gd3+) is toxic and, therefore, the ability of the ligand to bind tightly to the Gd ion is an important safety consideration.10 Moreover, since 2006, when an association was made between the development of NSF and the administration of Gd,11 stability has become an even greater concern in the selection of a GBCA.
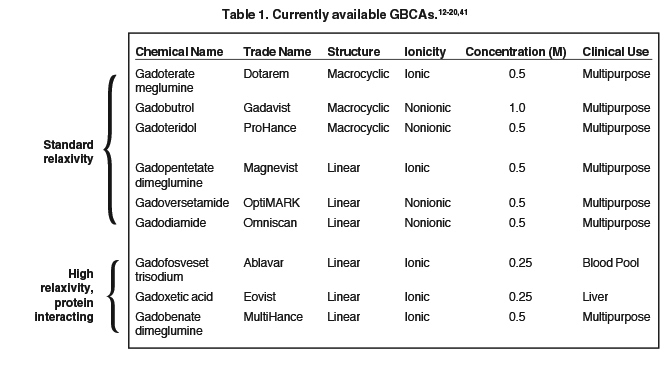
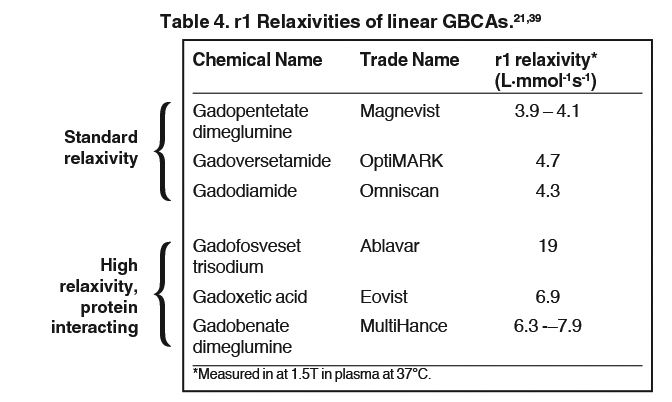
The 9 available GBCAs can be classified on the basis of relaxivity into 3 groups: macrocyclic standard relaxivity agents (including gadoterate meglumine [Dotarem],12 gadobutrol [Gadavist],13 and gadoteridol [ProHance]),14 linear standard relaxivity agents (gadopentetate dimeglumine [Magnevist]),15 gadodiamide [Omniscan],16 and gadoversetamide [OptiMARK]),17 and high-relaxivity agents, which interact with or overtly bind to proteins (the blood pool agent gadofosveset trisodium (Ablavar),18 the liver imaging agent gadoxetic acid (Eovist),19 and the multipurpose agent gadobenate dimeglumine (MultiHance)20 (Table 1). While all 3 of these high-relaxivity agents are linear in structure, there are some interesting observations regarding unconfounded NSF association – or lack thereof – with these agents that will be covered below.
Here we describe the differences among these 3 groups of GBCAs, including the advantages and disadvantages of their physicochemical profiles as they pertain to the selection of a GBCA. In addition, we summarize the published evidence surrounding the efficacy and safety of these 3 groups of agents. Finally, we discuss considerations when adopting a new GBCA for a radiology practice in order to maximize diagnostic yield while minimizing patient risk.
Macrocyclic GBCAs
Three macrocyclic agents are currently approved by the FDA: gadoterate meglumine (Dotarem), gadobutrol (Gadavist), and gadoteridol (ProHance). In terms of relaxivity, all are nonprotein binding and therefore standard relaxivity, with r1 relaxivities in the range of 4 to 5 L·mmol-1s-1.21 In terms of stability, macrocyclic agents utilize a closed, cage-like ligand that surrounds and binds tightly to the Gd ion, resulting in highly stable complexes (Table 2).10 Many published studies exist to support the high stability of these macrocyclic agents relative to the linear agents and, in terms of clinical value, these data can be considered to exist within a hierarchy, from in vitro, test-tube data at the bottom all the way up to human in vivo data at the top (Figure 1). When assessing the various data, it is important to recognize that higher-level data (eg, clinical experience) always trump data lower on the hierarchy (eg, test tube numbers).
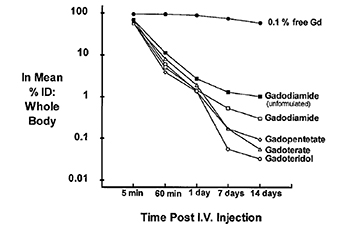
Test-tube data derived from GBCAs in solution demonstrate that as a class, the macrocyclic agents have high thermodynamic binding constants (log Ktherm and log Kcond [essentially log Ktherm measured at physiologic pH]) (Table 2).10 In vivo, the dissociation of GBCAs into gadolinium ion and ligand can be facilitated by a number of competing endogenous metals, such as zinc, copper, calcium, and iron, all of which may work simultaneously to destabilize the complex and lead to its dissociation. This displacement of the gadolinium ion from its ligand by other metals through competitive ionic binding is termed transmetallation (or dissociation or dechelation), and transmetallation has been studied both in vivo and in vitro. In vitro, copper and zinc ion stress test data demonstrate that in the presence of these competitors, the macrocyclic agents gadoterate meglumine (Dotarem) and gadoteridol (ProHance) remain essentially intact (<1% reaction), while the less stable linear agents gadodiamide (Omniscan) and gadopentetate dimeglumine (Magnevist) are more highly reactive.22 In vivo data from mice and rats also demonstrate that up to 14 days after injection of radiolabeled GBCAs, the lowest levels of residual Gd are observed with the macrocyclic agents23 (Figure 2).
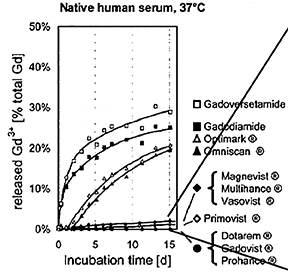
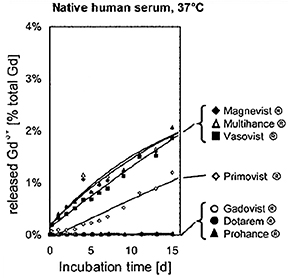
Human ex vivo data also demonstrate that transmetallation can occur with the low stability, linear agents gadodiamide (Omniscan) and gadoversetamide (OptiMARK). In this case, the data come from the observation that these 2 agents (and not the more stable agents) interfere in the colorimetric serum assay for calcium.24 The mechanism for this interference is believed to be removal of the Gd from the ligand-Gd complex by the specific dye used in the assay, preventing the dye from binding calcium.24 The result is a spurious hypocalcemia result for the patient, with potentially harmful clinical consequences. More recently, it was demonstrated that up to 20% of the nonionic linear GBCAs dissociate in human plasma up to 2 weeks after administration, while for the ionic linear agents and the macrocyclics, that number was closer to 2% and 0%, respectively (Figure 3).25 Human in vivo data, the most credible data, also exist to support the high stability of the macrocyclic agents: White and colleagues demonstrated 4 times more Gd3+ deposited in the bone of hip replacement patients after administration of gadodiamide (Omniscan) vs gadoteridol (ProHance).26
Taken together, all of these data suggest that transmetallation occurs both ex vivo and in vivo, and that with low stability linear GBCAs, there is more marked displacement of the gadolinium ion from its ligand by other metals. It is notable that the only GBCA with a triple dose indication is one of the macrocyclic agents, gadoteridol (ProHance)14 (the approval for triple dose indication for the linear agent gadodiamide [Omniscan] was withdrawn by the FDA in December, 2010).
It should also be noted that Pietsch and colleagues recently used a sensitive in vivo animal model to derive elimination time-courses for Gd in the skin of rats and found significantly higher nmolGd/g of skin at both Day 35 and Day 364 for the less stable agents compared with the macrocyclic agents (Table 3).27 However, a very small amount of Gd was still present in the skin of rats administered a macrocyclic agent on day 364, indicating that although the risk of dissociation with macrocyclic GBCAs is very low, this “low risk” is not equivalent to “no risk” and therefore, in patients at highest risk of NSF, it is still best to carefully assess the risk vs the benefit of injecting any GBCA.
Standard- and High-Relaxivity Linear Agents
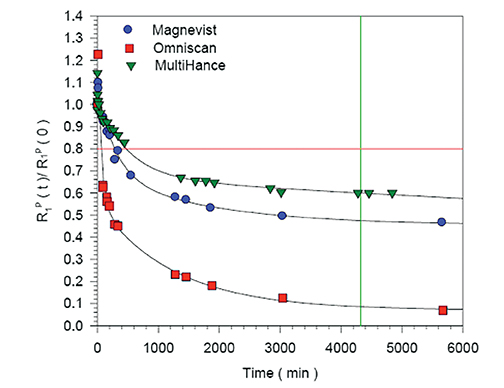
The linear agents can be divided into nonprotein binding, standard-relaxivity GBCAs and protein interacting/binding, high-relaxivity agents. Tables 2 and 4, respectively, show the stability and r1 relaxivity measurements for the various linear agents. The thermodynamic stability constant (Ktherm) is a measure of stability: a lower thermodynamic stability constant indicates that the Gd3+ ion will be more readily released.28 However, the thermodynamic stability constant does not take pH into account. The conditional stability constant (Kcond) is a measure of the stability of a complex at physiologic pH and, therefore, Kcond is considered a more relevant stability parameter. Note that the nonionic linear GBCAs gadodiamide (Omniscan) and gadoversetamide (OptiMARK) have the lowest conditional stability constants, and this low stability is believed to be related to the higher prevalence of NSF cases associated with these GBCAs.29 Based on in vitro Zn2+ transmetallation data (Figure 4), gadodiamide (Omniscan) has lower stability and gadobenate dimeglumine (MultiHance) has higher stability compared with gadopentetate dimeglumine (Magnevist).30 However, based on higher level, human ex vivo data, there is essentially no difference in the amounts of Gd3+ released among the ionic linear agents gadobenate dimeglumine (MultiHance), gadopentetate dimeglumine (Magnevist), and gadofosveset trisodium (Ablavar) when measured in native human serum at 37°C (Figure 3).25
How does protein interaction influence relaxivity? Interaction with serum proteins, most notably human serum albumin (HSA), greatly increases the effective size of the Gd-ligand complex, reducing its molecular motion, leading to greater T1 shortening and substantially increased signal-intensity enhancement.31 Gadofosveset trisodium (Ablavar), gadoxetate meglumine (Eovist), and gadobenate dimeglumine (MultiHance) all interact with HSA, the first strongly and the last 2 weakly.30
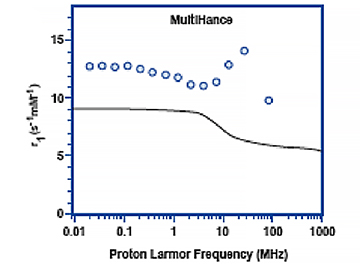
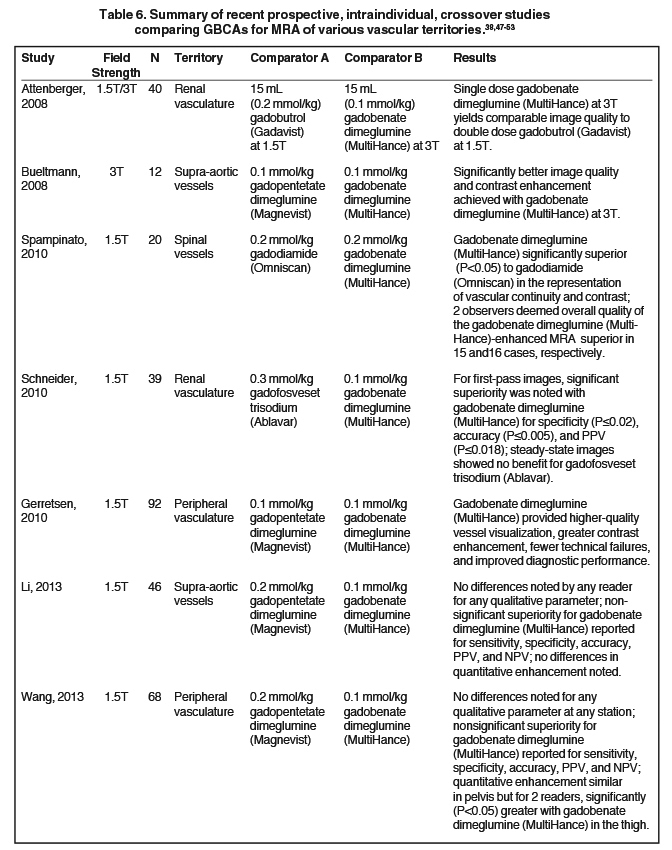
Figure 5 shows the relaxivity profiles of solutions containing 1mM of either gadobutrol (Gadavist) or gadobenate dimeglumine (MultiHance) in the absence and presence 4% HSA.30 Note that for gadobutrol (Gadavist), as well as for all other nonprotein binding GBCAs, the presence of HSA has no effect on the relaxivity profiles. On the other hand, for gadobenate dimeglumine (MultiHance), the presence of HSA results in a notable spike in measured relaxivity precisely in the range (≈10-150 MHz) of magnetic field strengths used in clinical practice (0.47T = 20 MHz; 3T = 127.5 MHz). It is this protein interaction, and resulting increased relaxivity, that likely explains results of intraindividual crossover studies demonstrating greater signal intensity and diagnostic performance for the high-relaxivity multipurpose GBCA gadobenate dimeglumine (MultiHance) vs other GBCAs in CNS,1-3 breast,32 and liver,33 as well as for MRA of various vascular territories.34-38
Clinical Considerations in GBCA Selection
Returning now to our 3 groups of agents, macrocyclic agents with standard relaxivity, linear agents with standard relaxivity, and linear agents with higher relaxivity, how does one select a GBCA for routine use applications?
Efficacy
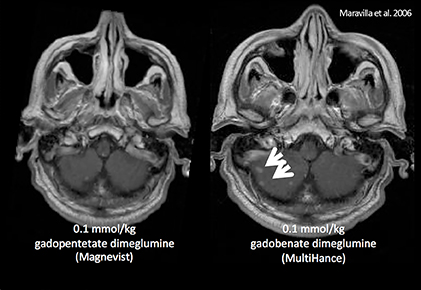
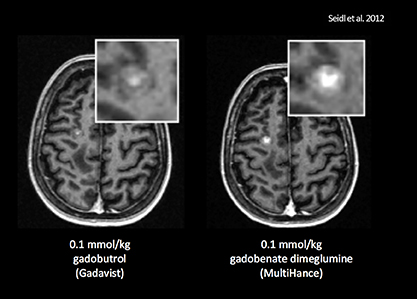
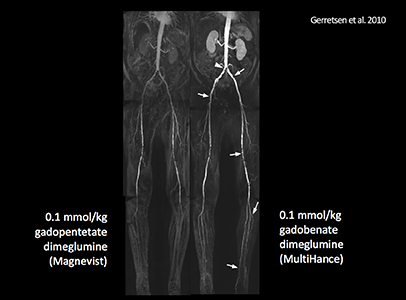
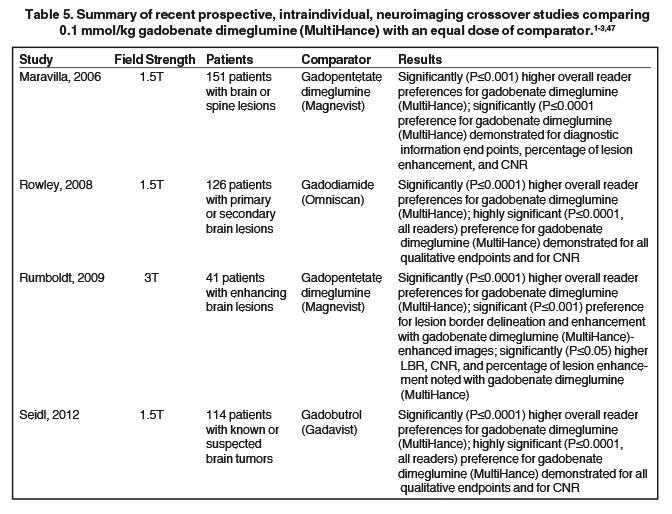
It has been well established that greater relaxivity leads to higher signal intensity enhancement. Of the multipurpose linear agents, gadobenate dimeglumine (MultiHance) has the highest r1 relaxivity at 1.5T, and this high relaxivity persists at 3T.39 Tables 5 and 6 summarize results from the most recent, robust intraindividual crossover studies comparing the efficacy of gadobenate dimeglumine (MultiHance) with standard relaxivity agents for MR imaging of the CNS (Table 5) and vasculature (Table 6). Examples of image pairs from published comparative studies are shown in Figure 6. From this body of evidence, one can conclude that at typical so-called T1-weighted imaging approaches, a single dose of gadobenate dimeglumine (MultiHance) is very roughly diagnostically equivalent to a double dose of a standard relaxivity agent, and that it provides better qualitative and quantitative diagnostic MR images when compared to an equivalent dose of a standard-relaxivity agent, for a variety of applications.
Safety
Regarding safety profiles, all of the GBCAs are considered to have low and comparable acute adverse event (AE) rates, regardless of whether the data are derived from product package inserts,12-20 from clinical trial safety data,40 or from professional society guidelines.41 The vast majority of AEs are mild, with the most common being transient injection site discomfort, nausea with or without vomiting, headache, paresthesia, dizziness, and itching.41
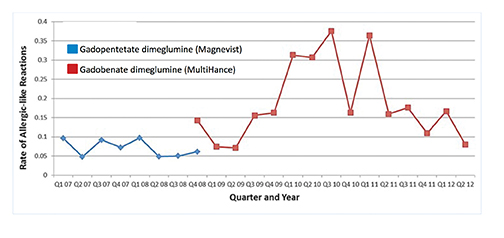
Upon adoption of a new drug, reported AE rates may go up initially but over time, tend to return to baseline. This was first observed by Weber in 1984 and has thus been termed the Weber effect.42 Davenport and colleagues observed the Weber effect at their institution when switching from gadopentetate dimeglumine (Magnevist) to gadobenate dimeglumine (MultiHance).43 Specifically, they noted that the reaction rates for gadobenate dimeglumine (MultiHance) peaked in the second year after it replaced gadopentetate dimeglumine (Magnevist), and then declined in the third year, ultimately returning to a baseline rate that did not differ significantly from the original baseline rate of gadopentetate dimeglumine (Magnevist) (Figure 7). The reasons for the Weber effect are complex and not completely understood, but it is generally believed to be related to unfamiliarity with a new drug, or new contrast agent in this case, and/or concern from staff regarding the change.
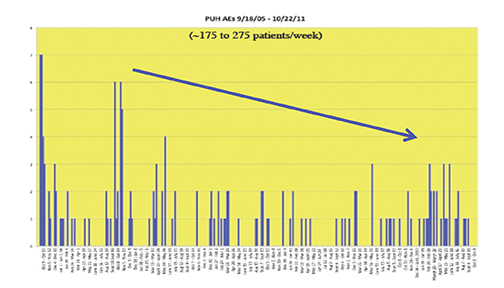
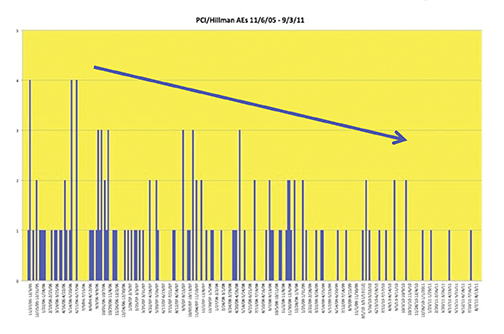
Recently, the incidence of AEs was prospectively studied at 10 sites associated with the University of Pittsburgh Medical Center (UPMC).44 A summary of the findings is shown in Table 7 and Figure 8. Most noteworthy is the gradual decline in AE rates over time, indicative of the Weber effect. Specifically, for data collected from 2005 through 2006, the overall AE rate was 178/23,553 (0.8%), but when the data collection was extended through 2011, the overall AE rate declined to 474/125,308 (< 0.4%).45 The incidence of serious AEs from this prospective study with gadobenate dimeglumine (MultiHance) was much lower (8/23,553 [0.03%]) and similar to the incidence of serious AEs with gadopentetate dimeglumine (Magnevist; 1/4,892 [0.02%]), obtained retrospectively, consistent with a lack of Weber effect for serious AEs. In addition, during this same time period, the incidence of anaphylactoid events at UPMC was 0.004% or approximately 4/100,000, a rate that is much lower than that observed with either high-osmolar iodinated contrast media (≈100/100,000) or low-osmolar iodinated contrast media (≈20/100,000).29
The most significant potential nonacute event associated with Gd exposure remains NSF. The factors most closely associated with the development of NSF include severe renal failure in the patient, exposure to multiple/high doses of a GBCA, and administration of a less stable GBCA.41,46 However, the majority of patients with severe renal failure administered multiple and/or high doses of a relatively unstable GBCA do not develop NSF, so it is likely that there remain unidentified factors that contribute to the development of NSF.
Per the American College of Radiology, GBCAs can be grouped into 3 groups according to their NSF risk (Figure 9).41 These groupings are based on the numbers of unconfounded, single-agent cases of NSF recorded for each agent. However, it is also interesting to examine the number of unconfounded NSF cases and the number of doses distributed, but to do so using the grouping system introduced in the introduction (Figure 10). When one does so, there are 2 apparent observations: first, and not surprisingly, the incidence of NSF with the macrocyclic agents is extremely low considering the number of administered doses and, second, that the number of NSF cases is surprisingly low in the group of intermediate-stability, protein interacting GBCAs, including gadofosveset trisodium (Ablavar), gadoxetate meglumine (Eovist), and gadobenate dimeglumine (MultiHance), considering their number of administered doses, suggesting perhaps that there is something protective about protein binding that mitigates or prevents the development of NSF. Any evidence for such protection, or any possible mechanism, remains to be established.
Conclusions
Chemical stability and in vivo relaxivity are the GBCA properties most relevant for selection of a contrast agent for MRI. Agents with a macrocyclic structure are the most highly stable and, as they do not bind to serum proteins, they all possess similar, relatively low relaxivity. Relatively lower stability linear agents may be of standard or high relaxivity. No cases of NSF have been reported after the prior unconfounded administration of any of the high-relaxivity protein interacting agents, the vascular imaging agent gadofosveset trisodium (Ablavar), the hepatic imaging agent gadoxetate meglumine (Eovist), or the CNS/multipurpose agent gadobenate dimeglumine (MultiHance). In terms of NSF, “low risk” does not appear to be equivalent to “no risk”; some level of caution is still warranted for all agents in the highest-risk patients.
References
- Maravilla KR, Maldjian JA, Schmalfuss IM, et al. Contrast enhancement of central nervous system lesions: multicenter intraindividual crossover comparative study of two MR contrast agents. Radiology. 2006;240:389-400.
- Rowley HA, Scialfa G, Gao PY, et al. Contrast-enhanced MR imaging of brain lesions: a large scale intraindividual crossover comparison of gadobenate dimeglumine versus gadodiamide. AJNR Am J Neuroradiol. 2008;29:1684-1691.
- Seidl Z, Vymazal J, Mechl M, et al. Does higher gadolinium concentration play a role in the morphologic assessment of brain tumors? Results of a multicenter intraindividual crossover comparison of gadobutrol versus gadobenate dimeglumine (the MERIT Study). AJNR Am J Neuroradiol. 2012;33:1050-1058.
- Pediconi F, Fraioli F, Catalano C, et al. Gadobenate dimeglumine (Gd-DTPA) vs gadopentetate dimeglumine (Gd-BOPTA) for contrast-enhanced magnetic resonance angiography (MRA): Improvement in intravascular signal intensity and contrast to noise ratio. Radiol Med (Torino). 2003;106:87-93.
- Prokop M, Schneider G, Vanzulli A, et al. Contrast-enhanced MR angiography of the renal arteries: blinded multicenter crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine. Radiology. 2005;234:399-408.
- Achenbach M, Figiel JH, Burbelko M, Heverhagen JT. Prospective comparison of image quality and diagnostic accuracy of 0.5 molar gadobenate dimeglumine and 1.0 molar gadobutrol in contrast-enhanced run-off magnetic resonance angiography of the lower extremities. J Magn Reson Imaging. 2010;32:1166-1171.
- Woodard PK, Chenevert TL, Sostman HD, et al. Signal quality of single dose gadobenate dimeglumine pulmonary MRA examinations exceeds quality of MRA performed with double dose gadopentetate dimeglumine. Int J Cardiovasc Imaging. 2012;28:295-301.
- Li Y, Li X, Li D, et al. Multicenter, intraindividual comparison of single-dose gadobenate dimeglumine and double-dose gadopentetate dimeglumine for MR angiography of the supra-aortic arteries (the Supra-Aortic VALUE study). AJNR Am J Neuroradiol. 2013;34:847-854.
- Wang J, Yan F, Liu J, et al. Multicenter, intra-individual comparison of single dose gadobenate dimeglumine and double dose gadopentetate dimeglumine for MR angiography of the peripheral arteries (the Peripheral VALUE Study). J Magn Reson Imaging. 2013;38:926-937.
- Tweedle MF. The ProHance story: the making of a novel MRI contrast agent. Eur Radiol. 1997;7 Suppl 5:225-30.
- Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-1108.
- Dotarem® (gadoteric acid) [product information]. Wayne, NJ: Guerbet; March 2013.
- Gadavist™ (gadobutrol) injection [prescribing information]. Wayne, NJ; Bayer HealthCare Pharmaceuticals; March 2011.
- ProHance® (gadoteridol) injection, 279.3 mg/mL [prescribing information]. Princeton, NJ: Bracco Diagnostics Inc.; revised September 2011.
- Magnevist® (brand of gadopentetate dimeglumine) injection [prescribing information].Wayne, NJ: Bayer HealthCare Pharmaceuticals; March 2012.
- Omniscan™ (gadodiamide) injection [prescribing information]. Princeton, NJ: Amersham Health Inc.; December 2010.
- OptiMARK® 0.5 mmol/mL (gadoversetamide injection) [prescribing information]. St. Louis, MO: Mallinckrodt Inc.; November 2010.
- Ablavar® (gadofosveset trisodium) [prescribing information]. North Billerica, MA: Lantheus Medical Imaging, Inc.; February 2011.
- Eovist® (gadoxetate disodium) [prescribing information]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; November 2011.
- MultiHance® (gadobenate dimeglumine) injection, 529 mg/mL [prescribing information]. Princeton, NJ; Bracco Diagnostics Inc.; July 2012.
- Rohrer M, et al. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715-724. 5.
- Tweedle MF, Hagan JJ, Kumar K, Mantha S, Chang CA. Reaction of gadolinium chelates with endogenously available ions. Magn Reson Imaging. 1991;9(3):409-15.
- Tweedle MF, Wedeking P, Kumar K. Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterate, and gadodiamide in mice and rats. Invest Radiol. 1995 Jun;30(6):372-80.
- Prince MR, Erel HE, Lent RW, Blumenfeld J, Kent KC, Bush HL, Wang Y. Gadodiamide administration causes spurious hypocalcemia. Radiology. 2003 Jun;227(3):639-46.
- Frenzel T, Lengsfeld P, Schirmer H, Hütter J, Weinmann HJ. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol. 2008;43:817-828.
- White GW, Gibby WA, Tweedle MF. Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Invest Radiol. 2006 Mar;41(3):272-8.
- Pietsch H, Pering C, Lengsfeld P, Walter J, Steger-Hartmann T, Golfier S, Frenzel T, Hütter J, Weinmann HJ, Sieber MA. Evaluating the role of zinc in the occurrence of fibrosis of the skin: a preclinical study. J Magn Reson Imaging. 2009 Aug;30(2):374-83. doi: 10.1002/jmri.21845.
- Ersoy H1, Rybicki FJ. Biochemical safety profiles of gadolinium-based extracellular contrast agents and nephrogenic systemic fibrosis. J Magn Reson Imaging. 2007;26:1190-1197.
- Idée JM, Port M, Robic C, Medina C, Sabatou M, Corot C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging. 2009;30:1249-1258.
- Laurent S, Elst LV, Muller RN. Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Med Mol Imaging. 2006;1:128-137.
- Giesel FL, von Tengg-Kobligk H, Wilkinson ID, et al. Influence of human serum albumin on longitudinal and transverse relaxation rates (r1 and r2) of magnetic resonance contrast agents. Invest Radiol. 2006;41:222-228.
- Martincich L, Faivre-Pierret M, Zechmann CM, et al. Multicenter, double-blind, randomized, intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for breast MR imaging (DETECT Trial). Radiology. 2011;258:396-408.
- Schneider G, Maas R, Schultze Kool L, et al. Low-dose gadobenate dimeglumine versus standard dose gadopentetate dimeglumine for contrast-enhanced magnetic resonance imaging of the liver: an intra-individual crossover comparison. Invest Radiol. 2003;38:85-94.
- Knopp MV, Schoenberg SO, Rehm C, et al. Assessment of gadobenate dimeglumine for magnetic resonance angiography: phase I studies. Invest Radiol. 2002;37:706-715.
- Knopp MV, Giesel FL, von Tengg-Kobligk H, et al. Contrast-enhanced MR angiography of the run-off vasculature: intraindividual comparison of gadobenate dimeglumine with gadopentetate dimeglumine. J Magn Reson Imaging. 2003;17:694-702.
- Prokop M, Schneider G, Vanzulli A, et al. Contrast-enhanced MR angiography of the renal arteries: blinded multicenter crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine. Radiology. 2005;234:399-408.
- Bueltmann E, Erb G, Kirchin MA, Klose U, Naegele T. Intra-individual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for contrast-enhanced magnetic resonance angiography of the supraaortic vessels at 3 Tesla. Invest Radiol. 2008;43:695-702.
- Gerretsen SC, le Maire TF, Miller S, et al. Multicenter, double-blind, randomized, intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for MR angiography of peripheral arteries. Radiology. 2010;255:988-1000.
- Pintaske J, Martirosian P, Graf H, et al. Relaxivity of gadopentetate dimeglumine (Magnevist), gadobutrol (Gadovist), and gadobenate dimeglumine (MultiHance) in human blood plasma at 0.2, 1.5, and 3 Tesla [erratum in Invest Radiol. 2006;41:859]. Invest Radiol. 2006;41:213-221.
- Shellock FG, Parker JR, Venetianer C, et al. Safety of gadobenate dimeglumine (MultiHance): summary of findings from clinical studies and postmarketing surveillance. Invest Radiol. 2006;41:500-509.
- American College of Radiology (ACR). ACR Manual on Contrast Media. 2013; version 9:81-98. Available at: http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/Contrast%20Manual/2013_Contrast_Media.pdf.
- Weber JCP. Epidemiology of Adverse Reactions to Nonsteroidal Antiinflammatory Drugs. New York, NY: Raven Press; 1984:1–7. Rainsford KD, Velo GP, eds. Advances in Inflammation Research; vol 6.
- Davenport MS, Dillman JR, Cohan RH, et al. Effect of abrupt substitution of gadobenate dimeglumine for gadopentetate dimeglumine on rate of allergic-like reactions. Radiology. 2013;266:773-782.
- Bleicher AG, Kanal E. Assessment of adverse reaction rates to a newly approved MRI contrast agent: review of 23,553 administrations of gadobenate dimeglumine. AJR Am J Roentgenol. 2008 Dec;191(6):W307-11.
- (E. Kanal, unpublished data).
- Thomsen HS. Nephrogenic systemic fibrosis: a serious late adverse reaction to gadodiamide. Eur Radiol. 2006;16:2619-2621.
- Rumboldt Z, et al. Multicenter, double-blind, randomized, intra-individual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine in MRI of brain tumors at 3 Tesla. JMRI. 2009;29:760-767.
- Attenberger UI, Michaely HJ, Wintersperger BJ, et al. Three-dimensional contrast-enhanced magnetic-resonance angiography of the renal arteries: interindividual comparison of 0.2 mmol/kg gadobutrol at 1.5 T and 0.1 mmol/kg gadobenate dimeglumine at 3.0 T. Euro Radiol. 2008;18(6):1260-8.
- Bueltmann E, Erb G, Kirchin MA, Klose U, Naegele T. Intra-individual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for contrast-enhanced magnetic resonance angiography of the supraaortic vessels at 3 Tesla. Invest Radiol. 2008;43:695-702.
- Spampinato MV, Nguyen SA, Rumboldt Z. Comparison of gadobenate dimeglumine and gadodiamide in the evaluation of spinal vascular anatomy with MR angiography. AJR Am J Neuroradiol. 2010;31:1151-1156.
- Schneider G, Maas R, Schultze Kool L, et al. Low-dose gadobenate dimeglumine versus standard dose gadopentetate dimeglumine for contrast-enhanced magnetic resonance imaging of the liver: an intra-individual crossover comparison. Invest Radiol. 2003;38:85-94.
- Li Y, Li X, Li D, et al. Multicenter, intraindividual comparison of single-dose gadobenate dimeglumine and double-dose gadopentetate dimeglumine for MR angiography of the supra-aortic arteries (the Supra-Aortic VALUE study). AJNR Am J Neuroradiol. 2013;34:847-854.
- Wang J, Yan F, Liu J, et al. Multicenter, intra-individual comparison of single dose gadobenate dimeglumine and double dose gadopentetate dimeglumine for MR angiography of the peripheral arteries (the Peripheral VALUE Study). J Magn Reson Imaging. 2013;38:926-937.
This article was published in a supplement to the May 2014 issue of Applied Radiology and was sponsored by an educational grant from Bracco.
