Confidently characterizing benign liver tumors

Benign liver tumors are exceedingly common and frequently encountered in daily clinical practice. And while their typical appearances pose no significant diagnostic challenge, the atypical appearances may pose difficulty, given their unfamiliarity to many radiologists. Also, an overlap in imaging characteristics in some cases may preclude the definitive differentiation between benign tumors or the confident exclusion of more ominous, malignant lesions. Therefore, understanding the common and uncommon appearances of frequently encountered benign liver tumors as well as the expected appearance of rarely seen tumors is important. Strategies for confidently diagnosing liver tumors or, in some cases, concluding that additional imaging or an invasive procedure is warranted, are equally vital.
While various methods of categorizing benign liver tumors are possible, a classification based on their cell of origin offers an intuitive,simple approach. Thus, we will consider benign liver tumors of epithelial and nonepithelial origin, with the epithelial tumors further classified into tumors of either cholangiocellular or hepatocellular origin. Of the nonepithelial tumors, those of mesenchymal cell origin will be considered.
Benign hepatic tumors of cholangiocellular origin
Hepatic cysts
Hepatic cysts are extremely common, identified on imaging in > 10% to 15% of the population, and their isolated, simple appearance typically poses no diagnostic difficulty.1 Simple hepatic cysts are epithelial lined; the epithelium is similar to that of the bile ducts. While their etiology has not been definitively established, isolated hepatic cysts are hypothesized to be hamartomatous tissue.2 Simple hepatic cysts are typically incidentally discovered, with rare complications including infection and cyst rupture.3
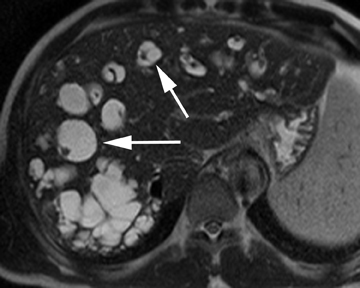
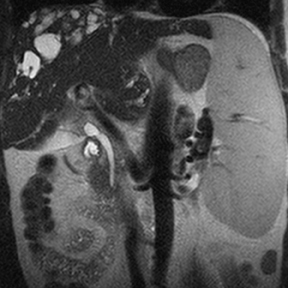
Hepatic cysts are also associated with polycystic liver diseases, which consist of a heterogeneous group of disorders of biliary epithelium,including autosomal-dominant polycystic kidney disease, isolated polycystic liver disease, and the fibropolycystic liver diseases.4 Patients with autosomal-dominant polycystic kidney disease (ADPKD) demonstrate multiple cysts in the kidney, liver, and pancreas, and while their liver is often enlarged with numerous cysts, function tends to be preserved. Isolated polycystic liver disease, which is far less common thanADPKD, is characterized by numerous hepatic cysts and an absence of renal cysts. Finally, the fibropolycystic liver diseases include various disorders, including congenital hepatic fibrosis, Caroli disease, autosomal recessive polycystic kidney disease (ARPKD), and biliary hamartoma. Of the fibropolycystic liver diseases, patients with congenital hepatic fibrosis and Caroli disease often develop recurrent acute cholangitis and portal hypertension secondary to peribiliary fibrosis (Figure 1). In addition, Caroli disease patients are at increased risk for developing cholangiocarcinoma (Figure 1).5 Biliary hamartomas, also referred to as von Meyenburg complex, are common, benign lesions characterized by the presence of numerous, small cystic lesions in the liver parenchyma ranging from 2 mm to 15 mm (Figure 2).6 On both computed tomography(CT) and magnetic resonance imaging (MRI), biliary hamartomas are characterized as small cystic lesions, the cystic nature of which may be more readily apparent on MRI; in some cases, uniform or peripheral enhancement of these lesions has been reported.7
Biliary cystadenoma
Biliary cystadenomas are rare, typically multilocular neoplasms of the bile ducts that are more frequently encountered in women.2 These predominantly cystic tumors frequently consist of proteinaceous fluid, and a thick capsule, internal septations, and areas of calcification maybe identified on imaging.8 In addition, nodular, enhancing soft-tissue components may be identified within these lesions, but do not definitively indicate the presence of a malignant cystadenocarcinoma. Given the significant overlap of imaging findings between biliary cystadenomas and malignant biliary cystadenocarcinomas, which precludes definitive differentiation, surgical consultation is typically warranted.
Intraductal papillary neoplasm of the bile ducts
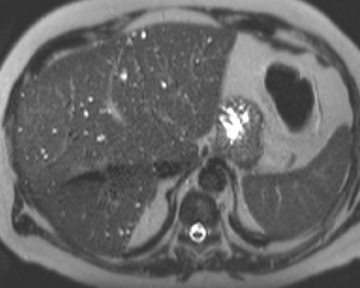
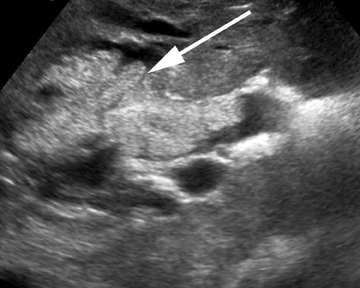
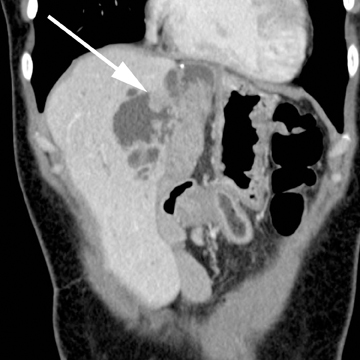
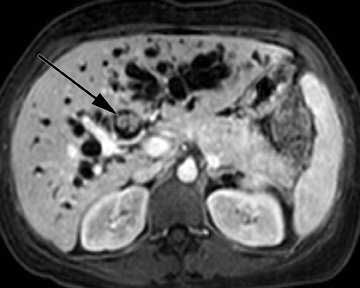
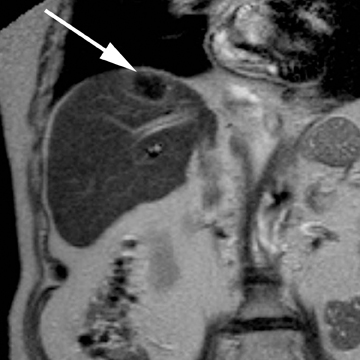
Intraductal papillary neoplasm (IPN) of the bile ducts encompasses a range of clinical and histologic features and is characterized by the presence of intraluminal tumors of the bile ducts, which often produce mucobilia as a distinctive feature (Figure 3).9 This entity parallels the more commonly encountered and familiar intraductal papillary mucinous neoplasm (IPMN) of the pancreas, in that the tumors range from benign to malignant, and are classified as adenoma, borderline tumor, carcinoma in situ, or carcinoma, though most cases are reportedly at least carcinoma in situ, a distinction from IPMN of the pancreas. On imaging, biliary dilatation is often present, related to the mucobilia, which is associated with this disease process, is often present on imaging. In addition, cystic intrahepatic mass lesions may be identified; in some cases, direct communication with adjacent intrahepatic bile ducts may also be identified on imaging.9
Peribiliary cysts
Peribiliary cysts represent retention cysts of peribiliary glands, identified along the course of the portal veins, predominantly in the centralliver.10 Peribiliary cysts are associated with advanced and portal hypertensive liver disease and are also present in patients with polycysticliver disease.11 Uncommon complications of peribiliary cysts include obstructive jaundice and hepatolithiasis. On imaging, peribiliary cysts may be misinterpreted as intrahepatic biliary dilatation, given their cystic nature and their presence along the bile ducts. Their central location as well as the direct visualization of their discrete cystic nature, which is typically apparent on MRI, offers useful imaging features in differentiating peribiliary cysts from biliary dilatation.
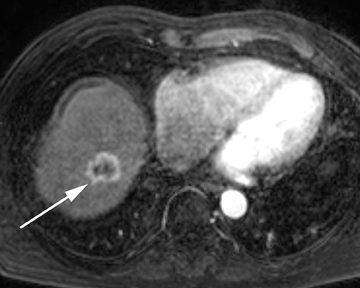
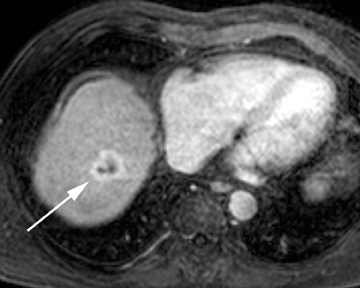
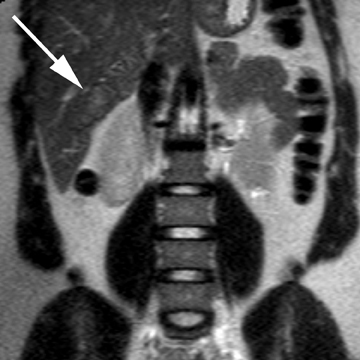
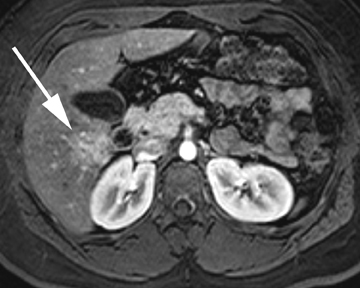
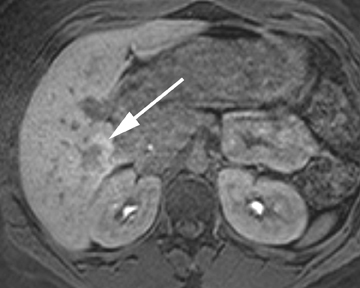
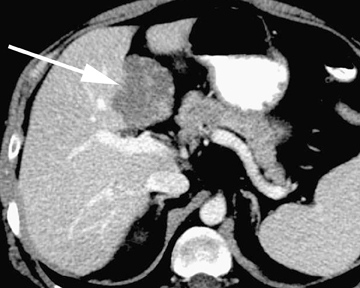
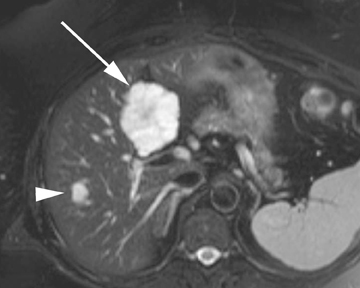
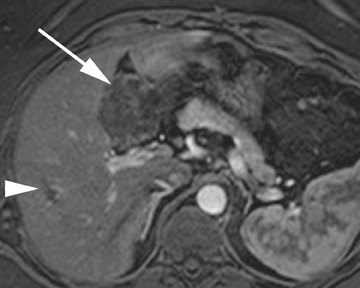
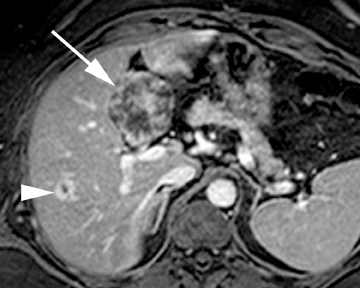
Intrahepatic bile duct adenoma
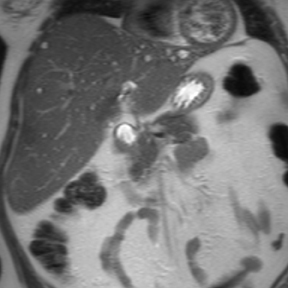
Intrahepatic bile duct adenomas are rare, benign tumors composed of disorganized but mature peribiliary gland acini and tubules. The etiology of these lesions remains uncertain, but they are thought to represent bile ductular reaction to a focal insult. On imaging, the lesions have been reported to be located in the periphery of the liver and to demonstrate early peripheral as well as delayed, persistent enhancement after contrast administration (Figure 4).12 On MRI, relative hypointensity on both T1- and T2-weighted images has been reported (Figure 4).13
Benign hepatic tumors of hepatocellular origin
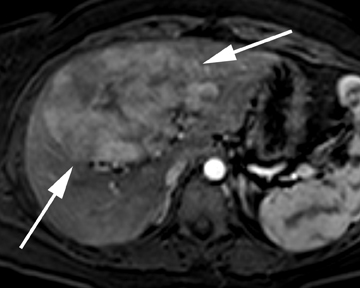
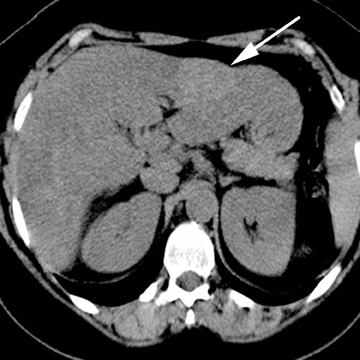
Focal nodular hyperplasia
Focal nodular hyperplasia (FNH) represents the second-most common benign tumor of the liver with a reported incidence of 0.9%.14 Typically an incidental finding on imaging studies, FNH is theorized to represent a hyperplastic response to a localized vascular abnormality, given its association with benign vascular neoplasms, including hemangiomas. The differentiation of FNH from other hepatocellular lesions, such as hepatic adenoma and hepatocellular carcinoma, is clinically important and may be confidently achieved with noninvasive imaging.
Unless a prominent central scar is identified, FNH is often subtle on ultrasound, with only minimal differences in echogenicity compared to the normal liver parenchyma. On contrast CT, FNH is characterized by brisk, homogeneous enhancement, save for the central scar (Figure 5). On subsequent, later phases of enhancement, the lesions typically become increasingly isoattenuating with respect to the adjacent liver parenchyma while the central scar may demonstrate enhancement, particularly on a more delayed phase of image acquisition. The presence of a central scar, a useful imaging feature in the confident diagnosis of FNH, has been reported in 50% of cases withCT imaging.15 Similar enhancement characteristics, including the central scar, have been reported on MRI. Compared to ultrasound and CT,MRI has both a higher sensitivity and specificity for FNH and detects the central scar more frequently.16 In cases in which a central scar is not readily identified or typical imaging features are not present, hepatocellular contrast agents may improve confidence in differentiatingFNH from other hepatocellular lesions (Figure 5). In the case of gadobenate dimeglumine, 97% of FNH are reported to be hyper- or isointense with respect to the adjacent liver parenchyma on the hepatocellular phase of image acquisition.17 In the case of gadoxetic acid, 100% ofFNH has been reported to be hyperintense with respect to the adjacent liver parenchyma on the hepatocellular phase of image acquisition.18 These contrast agents may increase reader confidence in the diagnosis or exclusion of FNH based on differences in enhancement patterns between this tumor and other hepatocellular lesions, discussed below.
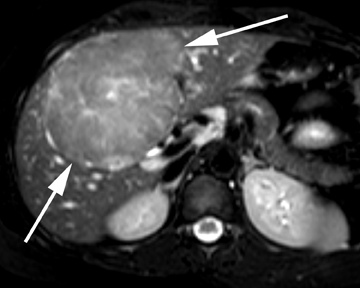
Hepatic adenoma
Hepatic adenomas are rare, benign liver tumors that are almost exclusively identified in young women taking oral contraceptives. In addition to estrogen-containing oral contraceptives, androgen-containing steroids and type I glycogen storage disease have been associated with hepatic adenomas.19 While typically solitary, multiple adenomas may be present; when > 10 adenomas are present, this is referred to as liver adenomatosis. Hepatic adenomas consist of hepatocytes arranged in sheets and cords instead of the typical lobular architecture, and they also include dilated sinusoids fed by prominent arteries.20 Feared complications of hepatic adenomas include a risk of hemorrhage, given the aforementioned tumoral architecture, as well malignant degeneration, and, therefore, their accurate diagnosis is of significant clinical importance.20
On ultrasound, hepatic adenomas are often echogenic with respect to the adjacent liver parenchyma, a finding that may reflect intratumorallipid or hemorrhage. Hemorrhage may also result in an increase in heterogeneity of the tumors on ultrasound.19 Intratumoral fat and hemorrhagemay be readily identified on CT, especially on unenhanced images. On contrast images, heterogeneous arterial enhancement is often identified,reportedly less marked compared to the brisk enhancement seen in FNH. On MRI, intratumoral fat may be readily assessed using chemical shift imaging, present in the overwhelming majority of cases in certain subtypes of hepatic adenoma assessed by MRI.21 Hepatic adenomas have mixed appearances on T1-weighted images, but they may be hyperintense secondary to the presence of hemorrhage or fat. On T2-weighted images, most hepatic adenomas are hyperintense with respect to the adjacent liver parenchyma (Figure 6).19 On contrast MR images, enhancement patterns similar to those described on CT are expected. The use of hepatocellular contrast agents offers additional data that may diagnose or exclude hepatic adenomas. With gadobenate dimeglumine, 100% of hepatic adenomas are reported to be hypointense with respect to the adjacent liver on the hepatocellular phase of image acquisition.17 However, with gadoxetic acid, hepatic adenomas have been reported to be hyperintensewith respect to the adjacent liver on the hepatocellular phase of image acquisition, which yields overlapping imaging features with FNH and precludes definitive differentiation in these cases.18,22 However, in cases in which hepatic adenomas are hyperintense with respect to the adjacent liver on the hepatocellular phase of image acquisition, the degree of hyperintensity has been reported to be useful in differentiating hepatic adenoma from FNH.22
Benign hepatic tumors of mesenchymal origin
Hemangioma
Hepatic hemangiomas are the most common solid, benign liver tumor; they consist of blood-filled spaces lined by endothelium, along with a variable presence of thrombi, calcification, fibrosis, and scarring. On unenhanced CT, relative hypoattenuation of hemangiomas are the expected appearance, though this may vary with the state of the surrounding liver parenchyma. For instance, in cases of significant hepatic steatosis, hepatic hemangiomas may be relatively hyperattenuating with respect to the adjacent, abnormally hypoattenuating liver parenchyma (Figure 7). On MRI, marked T2 hyperintensity is a useful, specific imaging feature of hepatic hemangiomas (Figure 8). On contrast imaging (CT as well as MRI), 3 general enhancement patterns may be expected, which somewhat depend on lesion size. In small hemangiomas, immediate uniform enhancement, so called “flash-filling,” may be observed.23 The most common pattern consists of a peripheral nodular enhancement that progresses centripetally over time to uniform enhancement on more delayed phases of image acquisition. Finally, especially in larger hemangiomas and giant hemangiomas (defined as having a diameter exceeding 5 cm), the early peripheralnodular enhancement may progress centripetally, but a persistent lack of uniform enhancement is seen on the more delayed phases of image acquisition.23 Additional atypical imaging features of hepatic hemangiomas include calcifications, a multilocular appearance, fluid-fluidlevels, hyalinization—which results in less marked T2 hyperintensity and a reduction in the degree of enhancement—and a pedunculated morphology, among others (Figure 8).23 The presence of hepatic hemangiomas is less common in cirrhotic livers; this is thought to be related to obliteration secondary to fibrosis. While hemangiomas in cirrhotic livers may pose a diagnostic dilemma given the increased incidence of hepatocellular carcinoma in this patient population, the expected imaging features of hemangiomas are typically present, allowing for confident diagnosis of this benign tumor.24
Lipoma/angiomyolipoma/myelolipoma
Benign, macroscopic fat-containing liver lesions of mesenchymal origin include hepatic angiomyolipomas and hepatic lipomas.25 Hepatic angiomyolipoma (AML) is a benign mesenchymal tumor composed of mature adipose tissue, thick-walled vessels, and smooth muscle. Any single component may dominate, and the relative composition of angiomyolipomas strongly dictates the imaging appearance.Compared to renal angiomyolipomas, there is a lesser degree of association between hepatic angiomyolipomas and tuberous sclerosis.26 Similar to renal angiomyolipomas, complications of hepatic AMLs include hemorrhage. The macroscopic fat hepatic AMLs may be readily identified on CT as well as on MRI with fat-suppression techniques, and brisk enhancement of the hepatic AMLs is often observed on contrast imaging.27 Hepatic lipomas are even rarer than hepatic angiomyolipomas; they consist of homogeneous macroscopic fat with expected,characteristic imaging features on both CT and MRI.25,28
Conclusion
Most benign liver tumors, even those of atypical appearance, may confidently be diagnosed with noninvasive imaging. Familiarity with the spectrum of imaging appearances of these exceedingly common tumors is useful in preventing misdiagnoses or inappropriate invasive interventions. A classification based on the cell of origin offers an intuitive approach to categorize the myriad benign hepatic tumors.
References
- Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol. 2003;58:626-629.
- Mortelé KJ, Ros PR. Cystic focal liver lesions in the adult: Differential CT and MR imaging features. Radiographics. 2001;21:895-910.
- Ishikawa H, Uchida S, Yokokura Y, et al. Nonparasitic solitary huge liver cysts causing intracystic hemorrhage or obstructive jaundice. J Hepatobiliary Pancreat Surg. 2002;9:764-768.
- Strazzabosco M, Somlo S. Polycystic liver diseases: Congenital disorders of cholangiocyte signaling. Gastroenterology. 2011;140:1855-1859.
- Bockhorn M, Malagó M, Lang H, et al. The role of surgery in Caroli’s disease. J Am Coll Surg. 2006;202:928-932.
- Lev-Toaff AS, Bach AM, Wechsler RJ, et al. The radiologic and pathologic spectrum of biliary hamartomas. AJR Am J Roentgenol. 1995;165: 309-313.
- Semelka RC, Hussain SM, Marcos HB, Woosley JT. Biliary hamartomas: Solitary and multiple lesions shown on current MR techniques including gadolinium enhancement. J Magn Reson Imaging. 1999;10:196-201.
- Lim JH, Jang KT, Rhim H, et al. Biliary cystic intraductal papillary mucinous tumor and cystadenoma/cystadenocarcinoma: differentiation by CT. Abdom Imaging. 2007;32:644-651.
- Lim JH, Zen Y, Jang KT, et al. Cyst-forming intraductal papillary neoplasm of the bile ducts: Description of imaging and pathologic aspects. AJR Am J Roentgenol. 2011;197:1111-1120.
- Terayama N, Matsui O, Hoshiba K, et al. Peribiliary cysts in liver cirrhosis: US, CT, and MR findings. J Comput Assist Tomogr. 1995;19: 419-423.
- Gupta S, Seith A, Dhiman RK, et al. CT of liver cysts in patients with autosomal dominant polycystic kidney disease. Acta Radiol. 1999;40: 444-448.
- Tajima T, Honda H, Kuroiwa T, et al. Radiologic features of intrahepatic bile duct adenoma: A look at the surface of the liver. J Comput Assist Tomogr. 1999;23:690-695.
- Maeda E, Uozumi K, Kato N, et al. Magnetic resonance findings of bile duct adenoma with calcification. Radiat Med. 2006;24:459-462.
- Wanless IR, Albrecht S, Bilbao J, et al. Multiple focal nodular hyperplasia of the liver associated with vascular malformation of various organs and neoplasia of the brain: Anew syndrome. Mod Pathol. 1989;2:456–462.
- Brancatelli G, Federle MP, Grazioli L, et al. Focal nodular hyperplasia: CT findings with emphasis on multiphasic helical CT in 78 patients. Radiology. 2001;219:61-68.
- Hussain SM, Terkivatan T, Zondervan PE, et al. Focal nodular hyperplasia: Findings at state-of-the-art MR imaging, US, CT, and pathologic analysis. Radiographics. 2004;24:3-17.
- Grazioli L, Morana G, Kirchin MA, Schneider G. Accurate differentiation of focal nodular hyperplasia from hepatic adenoma at gadobenate dimeglumine-enhanced MR imaging: prospective study. Radiology. 2005;236:166-177.
- Huppertz A, Haraida S, Kraus A, et al. Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT
- Grazioli L, Federle MP, Brancatelli G, et al. Hepatic adenomas: Imaging and pathologic findings. Radiographics. 2001;21:877-892.
- Micchelli ST, Vivekanandan P, Boitnott JK, et al. Malignant transformation of hepatic adenomas. Mod Pathol. 2008;21:491-497.
- Laumonier H, Bioulac-Sage P, Laurent C, et al. Hepatocellular adenomas: Magnetic resonance imaging features as a function of molecular pathological classification. Hepatology. 2008;48:808-818.
- Grazioli L, Bondioni MP, Haradome H, et al. Hepatocellular adenoma and focal nodular hyperplasia: Value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology. 2012;262:520-529.
- Vilgrain V, Boulos L, Vullierme MP, et al. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics. 2000;20:379-397.
- Brancatelli G, Federle MP, Blachar A, Grazioli L. Hemangioma in the cirrhotic liver: Diagnosis and natural history. Radiology. 2001;219:69-74.
- Basaran C, Karcaaltincaba M, Akata D, et al. Fat-containing lesions of the liver: Cross-sectional imaging findings with emphasis on MRI. AJR Am J Roentgenol. 2005;184:1103-1110.
- Hirasaki S, Koide N, Ogawa H, et al. Tuberous sclerosis associated with multiple hepatic lipomatous tumors and hemorrhagic renal angiomyolipoma. Intern Med. 1999;38:345-348.
- Hooper LD, Mergo PJ, Ros PR. Multiple hepatorenal angiomyolipomas: Diagnosis with fat suppression, gadolinium-enhanced MRI. Abdom Imaging. 1994;19:549-551.
- Martí-Bonmatí L, Menor F, Vizcaino I, Vilar J. Lipoma of the liver: US, CT, and MRI appearance. Gastrointest Radiol. 1989;14:155-157.
