Compression elasticity imaging of the breast: An overview
Images



Dr. Grajo is a Diagnostic Radiology Resident at the University of South Florida, Tampa, FL, and Dr. Barr is a Radiologist at Southwoods Radiology, Radiology Consultants Inc., Youngstown, OH.
One out of every 8 women will develop breast cancer during their lifetime. Currently, the best method of treating breast cancer involves early detection. Physicians have used palpation for many years as a way to detect lumps or nodules in the female breast. In fact, regular breast self-examinations (BSE) and clinical breast examinations (CBE) have long been used to detect breast nodules in their earliest stages of development. Although the effects of BSE on mortality rates from breast cancer are debatable, CBE still exists as a standard screening protocol for breast cancer in women. As an adjunct to direct manual palpation, physicians have utilized ultrasonography to visualize lesions in breast tissue. Lesion features are frequently characterized based on shape, echogenicity, shadowing, margin irregularity, and microlobulation.1-3 Ultrasound has been extremely important because of its ability to help differentiate cystic breast lesions from solid lesions. This provides the clinician with useful insight into the possible benignity or malignancy of a given lesion.
Over the past two decades, various new methods have been developed to sonographically evaluate the stiffness of a lesion.4-11 These methods offer the potential to quantify the formerly qualitative-only measurement of tissue stiffness; therefore, allowing for increased differentiation of cystic and solid breast lesions. With the use of standard ultrasound equipment and new “elasticity imaging” (EI) software, the trained clinician can now noninvasively differentiate benign and malignant breast lesions with high sensitivity and specificity. Although the standard of care for the diagnosis of breast cancer still involves biopsy, elastography has the potential to significantly reduce the number of biopsies of benign breast lesions.12,13 This would help to alleviate anxiety in many patients and to decrease the related high healthcare costs in the United States (U.S.). This article will review the physics behind the elastographic technique, the utility of elasticity imaging in clinical practice, the implications of EI on patient care, and possible advances in elastography in the near future.
Defining the technique
Elasticity imaging, also referred to as elastography, offers great potential to characterize cystic and solid breast lesions using a combination of standard ultrasound imaging and innovative software technology. There are 2 main categories of elasticity imaging: compression, or “strain,” elastography, and shear wave elastography. Strain elastography is a qualitative method that measures stiffness based on soft-tissue distortion caused by minimal manual compression. Conversely, shear wave elastography is a quantitative method that relies on the principles of Young’s modulus to measure the speed of low-frequency shear wave transmission through soft tissue. In the authors’ lab, minimal manual compression is utilized, usually provided by the patient’s respiratory cycle and cardiac rebound, to produce strain images. This article describes this method.
Like conventional B-mode sonographic imaging, EI differentiates cystic breast lesions from solid breast lesions. An artifact that occurs with some manufacturers’ equipment has been reported to characterize cystic lesions with high accuracy. The technology can also further characterize solid lesions that contain both benign and malignant elements. In such cases, the clinician can aspirate or biopsy the various elements in a lesion with the guidance of the elasticity software. This allows for obtaining appropriate breast tissue more accurately and helps to provide the pathologist with information regarding the likely composition of the specimen.
Besides characterizing the composition of breast lesions, EI offers the potential to differentiate benign lesions from malignant with high sensitivity and specificity. The technique is based on the differing physical properties of benign and malignant lesions, as described by clinicians throughout the literature. It has long been reported that benign lesions tend to be “softer,” with more mobility within the breast parenchyma, while malignant lesions tend to be “harder,” with a propensity for remaining firmly fixed within the tissue.14,15 The theory behind elastography is that cancerous and noncancerous lesions will demonstrate differing amounts of tissue motion relative to the normal surrounding breast parenchyma when minimal pressure is applied. The ability to differentiate soft and hard lesions allows the clinician to utilize EI to predict the benignity or malignancy of breast lesions. This would potentially lead to a significant decrease in the number of benign breast biopsies and, therefore, reduce the overall cost of care.
The physics of elastography
Compression EI is performed with a standard high-frequency ultrasound probe. The returning radiofrequency signals are analyzed in real time with the standard B-mode algorithm and the EI algorithm. The EI algorithm analyzes the stiffness of a lesion compared to the compressibility of its surrounding tissue, much like measuring the degree of stiffness of a marble suspended within a bowl of gelatin. In this model, external compression will cause the gelatin, and not the marble, to change shape. The computer analyzes this degree of deformation to determine if the lesion is soft or hard. Both the standard B-mode image and the EI image are displayed in real time. The EI algorithm is sensitive for a 0.1% strain and uses temporal persistency strategy to enhance the descriptive pattern in the elastogram. Using this technique in the breast, the elastography contrast for cancerous and noncancerous lesions is between 1000% and 5000%, as opposed to the tissue contrast of 1% to 100% between cancerous and noncancerous lesions using conventional imaging techniques, such as ultrasound, mammography, and magnetic resonance imaging (MRI).16 Motion is provided by patient breathing and cardiac rebound. If additional compression is required, slow minimal palpation with the probe can be applied. In patients with small breasts, the patient may have to hold her breath to limit motion. The sonographer must ensure that the lesion remains in the image plane during the compression cycle. It is important to have the probe and lesion perpendicular to the scanning table. We record a short clip that demonstrates both the compression and release stages. The best image is selected by cine review for subsequent measurements.
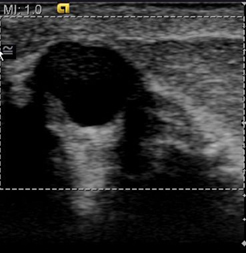
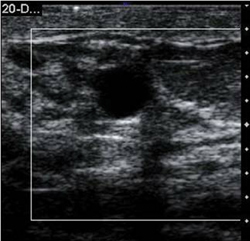
While performing an ultrasound on a patient with a known breast lesion(s), both the standard B-mode image and the elastogram are displayed side-by-side (Figure 1). We can then directly compare the relative stiffness of a lesion based on its elastic properties in relation to the surrounding tissue. In our study, we set the ultrasound machine to display “soft lesions” as white and “hard lesions” as black. This gives us immediate insight into the relative stiffness of any lesion within the breast parenchyma. It is important to emphasize that the elastographic technique provides the clinician only with the relative stiffness of breast lesions within the surrounding tissue. Therefore, lesions will exhibit varying shades of gray in fatty breast tissue as opposed to dense breast tissue. To obtain an appropriate dynamic range for interpretation, we try to include fatty tissue, normal dense breast tissue, and the lesion in the field of view. This limits our ability to utilize EI in a mass screening protocol.
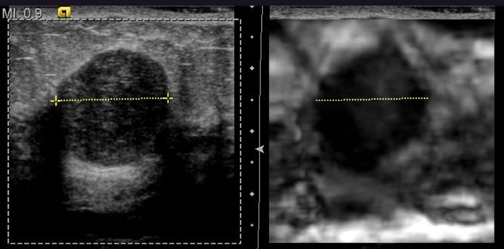
By displaying the B-mode sonographic image and the elastogram side-by-side, we can also directly compare the relative dimensions of the lesions in both image displays. We then predict the benignity or malignancy of the breast lesion based on size of the lesion in the elasticity image compared to its size in the B-mode display. Either length or area of the lesion in the greatest dimension can be utilized to measure the magnitude of the lesion. If the lesion appears smaller on the elastogram based on direct measurement, it is characterized as benign. If the lesion measures equally or larger on the elastogram compared to the B-mode image, the lesion is deemed to be malignant. In our initial study, we determined that EI is able to correctly differentiate benign and malignant breast lesions with very high sensitivity and specificity.17-19
Clinical utility of breast elasticity imaging
Elasticity imaging offers the potential to characterize various breast lesions in great detail and to provide the sonographer with an extremely helpful adjunct to the standard ultrasound examination. The elastographic technique is entirely noninvasive and adds only a few minutes to the conventional sonographic exam. It augments the value of the sonogram and allows for immediate interpretation of results. In addition, elastography provides the clinician with detailed characterization of the cystic or solid breast lesion that may not be readily appreciated on B-mode imaging. For example, lesions that appear to be solid on B-mode imaging may actually be complicated cysts when imaged with elastographic software. In the authors’ clinical study, 4% of all lesions that appeared solid on B-mode were actually complicated cysts when viewed as a strain image.20 These lesions can be easily aspirated, negating the need for subsequent biopsy.
The clinician can rapidly predict the benignity or malignancy of breast lesions with EI software and standard B-mode imaging techniques. In the authors’ clinical study, the size of the breast lesion on the B-mode and the elasticity image was directly compared. The lesion was determined as likely to be benign or malignant based on the ratio of the lesion’s size on the strain image compared to its size on B-mode image. If the ratio is <1, the lesion appears smaller on the elastogram. We predict that this lesion is most likely going to be benign when biopsied for pathologic correlation. On the other hand, the lesion is likely to be malignant if it appears larger on the elastogram compared to the standard B-mode image. The resulting ratio produced from direct measurement would be ≥1. Direct measurement of the lesion’s size in both the B-mode image and the elastogram can be measured in seconds. In fact, the size discrepancy between the images is sometimes so drastic that it can simply be “eyeballed” to immediately determine a relative ratio. Although biopsy of all lesions is still the standard of care for diagnosing breast lesions, the information garnered from the elastographic technique can augment the information that the radiologist can provide the pathologist. If the authors’ results can be duplicated on a large-scale, multicenter basis, it is hoped, then the initial determination of a breast lesion’s nature can lead to a decrease in the number of benign breast biopsies.
Appearance of benign lesions
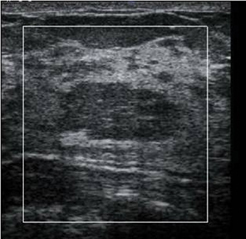
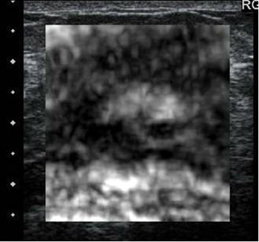
In our experience with EI, benign lesions appear smaller on strain images compared to the corresponding B-mode image (Figures 2 and 3). This is based on the relative elasticity of a breast lesion within its surrounding parenchyma. If a lesion demonstrates stiffness similar to normal dense breast tissue, it is more likely to be benign (Figure 4). In our lab, we also display soft structures as white, which further demarcates areas of increased elasticity. Of particular interest is the easy identification of both simple and complicated cysts with compression elastography. We have shown that cystic lesions demonstrate a “bull’s-eye” artifact, featuring a dark structure with both central and posterior bright foci (Figure 5).21 The identification of lesions as benign cystic lesions on elastography can lead to direct aspiration of symptomatic lesions and avoidance of biopsy for complicated cysts, which may otherwise appear suspicious on standard B-mode imaging. In lesions with both cystic and solid components, elasticity can help guide biopsy to obtain the most useful specimen.
Appearance of malignant lesions
Due to their increased stiffness, malignant lesions appear the same or larger on the elastogram when compared to the B-mode image. In our center, these malignant lesions appear dark on the strain images and may demonstrate adjacent areas of tissue invasion that would not be appreciated on B-mode. In the authors’ experience, we can predict malignancy based on the real-time elastogram with high confidence. Furthermore, a recent retrospective review has shown the potential to determine the aggressiveness of malignant lesions and predict the grade of these tumors via EI. Limited data analysis has demonstrated that more aggressive malignancies, such as invasive ductal carcinomas (Figures 6 and 7) and invasive lobular carcinomas (Figure 8), will exhibit higher elasticity/B-mode (E/B) ratios compared to mucinous carcinomas or ductal carcinoma in situ (DCIS), for example. Moreover, initial results demonstrate that higher grades of IDC correlate with higher E/B ratios in a statistically significant fashion.22 This observation could have important implications for the radiologist’s ability to characterize breast lesions, such as in classification according to the BIRADS system. With the use of elastography, one may be able to downgrade or upgrade BIRADS 3 and 4A lesions. Further validation and standardization of this technique is required.
Conclusion
Elasticity imaging has demonstrated the ability to differentiate benign and malignant breast lesions with high sensitivity and specificity. Numerous imagers have utilized various forms of the elastographic technique, including color elastography, strain ratio measurements, and, most recently, shear wave technology, to characterize an array of breast lesions. In our practice, the authors have produced very favorable results with compression elasticity images utilizing simple cardiac and respiratory cycles to provide the appropriate degree of compression for the production of strain images. The appropriate degree of compression is, in fact, a critical aspect of reproducing these favorable results. Applying excessive manual compression to breast tissue will compact the parenchyma and make fat appear harder, skewing the interpretation of the strain image. The concept of applying only minimal compression, termed “pre-compression,” is of vital importance in obtaining accurate and reproducible strain images.
EI can be particularly helpful when working up complicated breast lesions. Some lesions may contain both benign and malignant-appearing components, making it difficult to decide where to biopsy within the lesion. Because of the distinction between “soft” and “hard” lesions provided by elastography, the clinician can direct the biopsy needle to the more suspicious components within a particular breast lesion. The hard component of the lesion can be biopsied, while the soft component can be aspirated with FNA. This will subsequently lead to direct needle placement and increased biopsy accuracy. It will also enhance pathologic diagnosis, as the radiologist can provide the pathology lab with more detailed information along with the specimen itself.
Over the last several years, we have conducted both single-center and multicenter trials to demonstrate the effectiveness of compression elasticity imaging in differentiating benign and malignant breast lesions. With strain elastography, it is often difficult to interpret the elastogram in cases where a benign lesion (eg, fibroadenoma, fibrocystic change) lies within a background of dense breast tissue. The elastographic properties are similar and it is difficult to determine the boundaries of the lesion. Shear wave imaging will be helpful in these cases. We believe that strain elastography can have several implications for the future of breast imaging. These include a decrease in number of benign biopsies, a reduction in the costs of lesion work-up, incorporation into the BIRADS classification system, and possibly the prediction of breast cancer grade. Additionally, the elastographic technique can help direct needle biopsy and provide useful diagnostic information to the pathologist. As such, EI may prove to be an important adjunct to standard B-mode sonography in the evaluation of a breast lesion.
references
- Stavros AT, Thickman D, Rapp CL, et al. Solid breast nodules: Use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123-134.
- Velez N, Earnest DE, Staren ED. Diagnostic and interventional ultrasound for breast disease. Am J Surg. 2000;180:284-287.
- Dennis MA, Parker SH, Klaus AJ, et al. Breast biopsy avoidance: The value of normal mammograms and normal sonograms in the setting of a palpable lump. Radiology. 2001;219:186-191.
- Chaturvedi P, Insana MF, Hall TJ. Ultrasonic and elasticity imaging to model disease-induced changes in soft-tissue structure. Med Image Anal. 1998;2:325-338.
- Garra BS, Cespedes EI, Ophir J, et al. Elastography of breast lesions: Initial clinical results. Radiology. 1997;202:79-86.
- Ophir J, Cespedes I, Ponnekanti H, et al. Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111-134.
- Lubinski MA, Emelianov Y, O’Donnell M. Adaptive strain estimation using retrospective processing. IEEE Trans Ultrason Ferroelect Freq Control. 1999;46:97-107.
- Skovoroda AR, Emelianov Y, O’Donnell M. Tissue elasticity reconstruction based on ultrasonic displacement and strain images. IEEE Trans Ultrason Ferroelect Freq Control. 1995;42:747-765.
- Parker KJ, Fu D, Graceswki SM, et al. Vibration sonoelastography and the detectability of lesions. Ultrasound Med Biol. 1998;24:1437-1447.
- Taylor LS, Porter BC, Rubens DJ, Parker KJ. Three-dimensional sonoelastography: Principles and practices. Phys Med Biol. 2000;45:1477-1494.
- Hall TJ, Zhu Y, Spalding CS, Cook LT. In vivo results of real-time freehand elasticity imaging. In: Ultrasonics Symposium. 2001;1653-1657.
- Liberman L, Feng TL, Dershaw DD, et al. US-guided core breast biopsy: Use and cost-effectiveness. Radiology. 1998;208:717-723.
- Svensson WE et al. Elasticity imaging of 67 cancers and 167 benign breast lesions shows that it could halve biopsy rates of benign lesions. Proceedings of the 4th International Conference on the Measurement and Imaging of Tissue Elasticity. 2005:87.
- Emerson K. Diseases of the Breast. In: Wintrobe MM, Harrison TR, eds. Harrison’s Principles of Internal Medicine. Harrison’s Principles of Internal Medicine, 7th ed. New York: McGraw-Hill; 1974:582-587.
- Pruthi S. Detection and evaluation of a palpable breast mass. Mayo Clin Proc. 2001;76:641-647.
- Krouskop TA, Wheeler TM, Kallel F, et al. Elastic moduli of breast and prostate tissue under compression. Ultrason Imaging. 1998;20:260-274.
- Barr RG. Initial results of real-time elasticity of the breast. Proceedings of the Radiological Society of North America, 92nd Scientific Assembly and Annual Meeting. 2006.
- Barr RG, Grajo JR. Initial results of real-time elasticity imaging in the evaluation of breast lesions. Proceedings of the Sixth International Conference on the Ultrasonic Measurement and Imaging of Tissue Elasticity. 2007.
- Grajo JR, Barr RG. Elasticity imaging of the breast: A clinical perspective. Proceedings of the Radiological Society of North America, 93rd Scientific Assembly and Annual Meeting. 2007.
- Barr RG, Lackey AE. The utility of the “bull’s-eye” artifact on breast elasticity imaging in reducing breast lesion biopsy rate. Ultrasound Quarterly 2011;27:151-155.
- Barr RG, Grajo JR. Sensitivity and specificity of the “bull’s-eye” artifact on breast elasticity imaging to characterize cysts. Proceedings of the Radiological Society of North America, 94th Scientific Assembly and Annual Meeting. 2008.
- Grajo JR, Peterson CM, Barr RG. Does the EI/B-mode length ratio predict breast cancer tumor grade? Proceedings of the Ninth International Conference on the Ultrasonic Measurement and Imaging of Tissue Elasticity. 2010.