Challenges and pitfalls in postoperative spine imaging
Images
Dr. Hancock is a Neuroradiology Fellow, and Dr. Quencer is Chairman, Department of Radiology, and The Robert Shapiro M.D. Professor of Radiology, Jackson Memorial Hospital, University of Miami Hospital and Clinics, Miami, FL. Dr. Falcone is the Executive Clinical Dean, Florida Atlantic University, Boca Raton, FL.
Image interpretation of the postoperative spine presents significant difficulty because of the various types of surgery performed, new surgical devices employed, different imaging techniques used, and limited clinical information available at the time of image interpretation. The radiologist must understand the many different postoperative spine imaging presentations in order to accurately convey clinically important but often subtle findings.
In this article, we will discuss and illustrate some of the common and uncommon imaging findings in the postoperative spine, with a particular emphasis on the uncommon cases—specifically, retained surgical material with associated clinical sequelae, including gel foam, dural autograft, and catheters. The article will also address magnetic susceptibility artifacts and dislodged surgical hardware, as well as how to distinguish between inflammation and infection and between scar and recurrent disc herniation. Another topic covered is the common dilemma of delineation between postoperative fluid collections (including seroma, abscess, and pseudomeningocele). This article reviews several manifestations of postprocedure and postoperative spine imaging with a focus on the challenges and pitfalls that are often encountered.
Historical background
Spine surgery was first contemplated by the ancient Egyptians and was later advanced by the Greeks and Romans.1 Many of their works were preserved during the Dark Ages by Arabic and Byzantine translators and were rediscovered during the Renaissance.1 Major impediments to progress from Hippocrates’ era until the 19th century included the lack of antiseptics, inadequate anesthetics, and the absence of medical imaging. With the development of radiography, it was possible to visualize the effects of surgery and the consequences of intervention, and to document the presence of disease. The first successful spinal fusion was performed in 1911 to reduce pseudoarthrosis. This was followed by autologous interbody bone grafting in 1933; transfacet fusions in the 1940s; distraction Knodt rods in the 1950s; Harrington rods, methylmethacrylate, and transverse process plate fusion in the 1960s; Luque rods and sublaminar wire in the 1970s; and intrapedicle screws with rod fixation in the 1980s.2 A medical industrial complex surrounding spinal surgery has arisen as a direct result of the introduction of computed tomography (CT) and spinal magnetic resonance imaging (MRI).3 This growth in surgical intervention has in turn created an increase in the demand for advanced imaging for preoperative planning, intraoperative evaluations, immediate postsurgical assessment, and postoperative care. Postoperative evaluation of potential complications (such as retained surgical material, infection, pseudomeningocele, seroma, hemorrhage, loose hardware, compromised hardware, fracture, and pseudoarthrosis) plays an increasingly important role in the radiologist’s daily interpretation of the postoperative spine. Multiple artifacts may complicate the interpretation of spine imaging, and these artifacts can arise from chemical shift, motion, magnetic field nonuniformity, magnetic susceptibility and incomplete fat saturation.4 A textbook would be required to provide a complete compendium of postoperative imaging findings; therefore, this article includes several of the more interesting and challenging presentations.
Clinical considerations and imaging
Retained foreign material
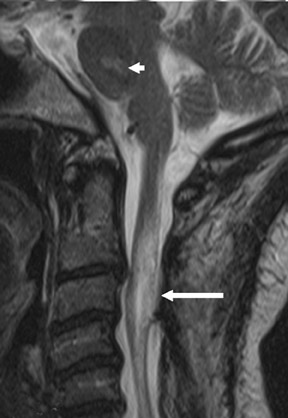
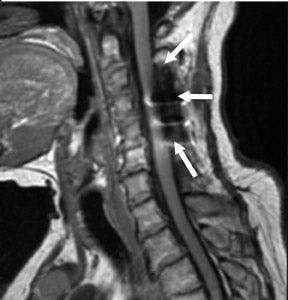
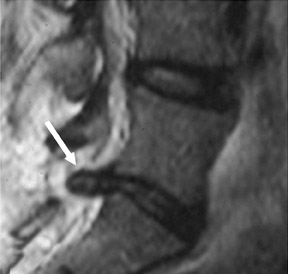
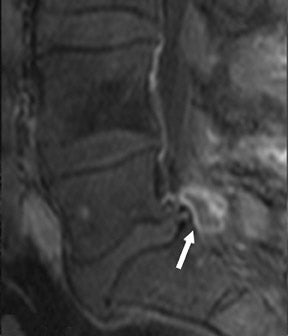
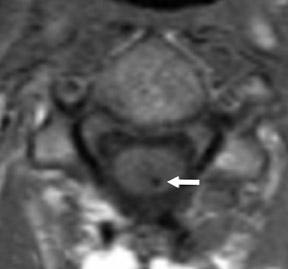
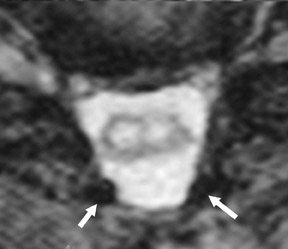
Gel foam—Placement of surgical hardware to obtain spinal stability typically leads to a successful postoperative outcome; however, surgical material may be unintentionally malpositioned or inadvertently retained, which may have significant patient care implications (Figure 1). Gel foam, which is used for hemostasis and postoperative scar reduction in spinal surgery, has been associated with significant pain.5
Pantopaque retention
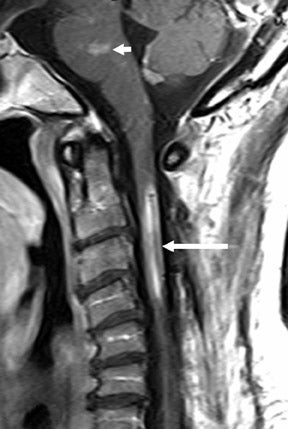
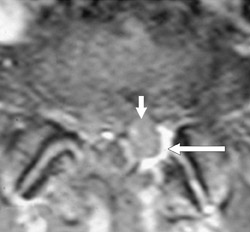
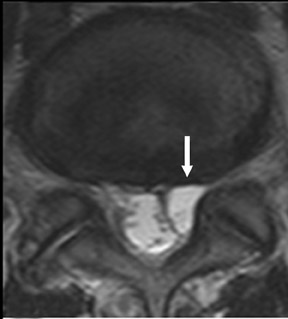
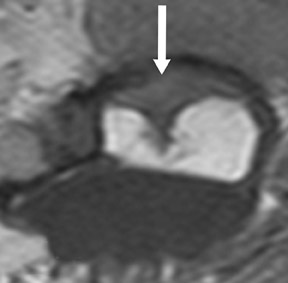
The myelographic agent that was used prior to the availability of water-soluble agents (Pantopaque, Lafayette Pharmaceutical, Inc., Lafayette, IN) could be retained within the spinal canal for years or even decades, depending on the volume used.6 Adhesive arachnoiditis may result as a complication of persistent Pantopaque (Figure 2), which can cause meningeal thickening and hyalinization of the arachnoid.7 The nerve roots may adhere to themselves and/or the dural margins.8 Arachnoiditis may occur in the postmyelographic state, particularly if bloody cerebrospinal fluid (CSF) is present.
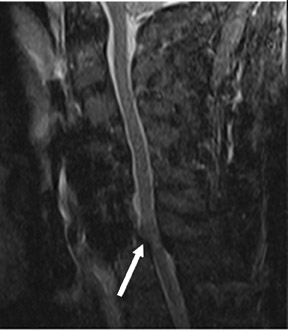
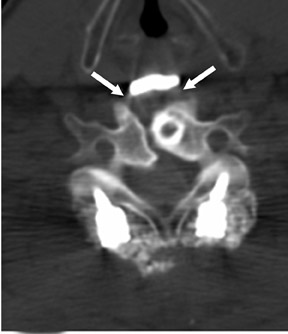
Catheters and stents
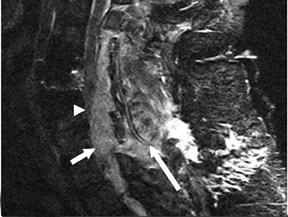
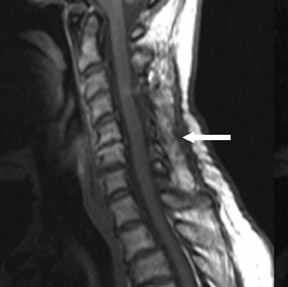
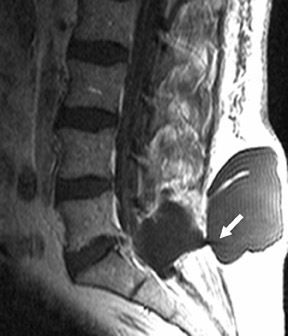
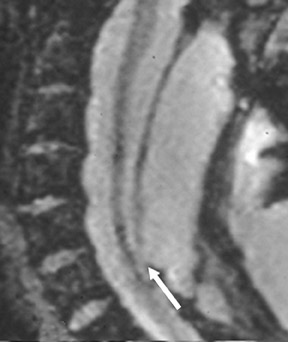
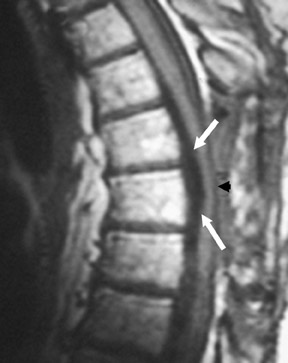
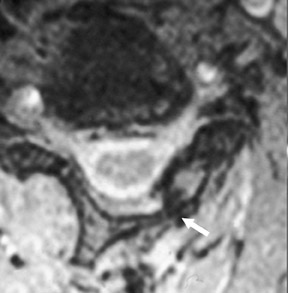
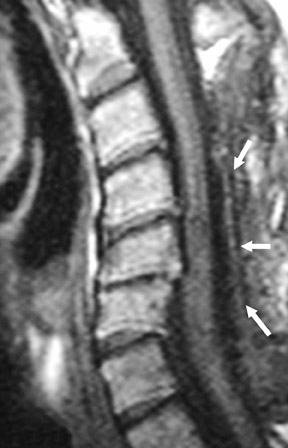
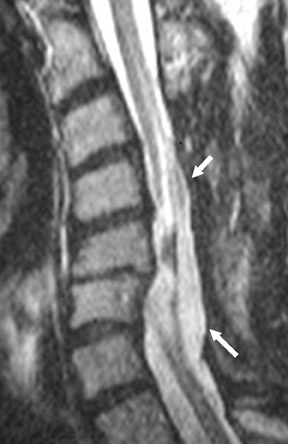
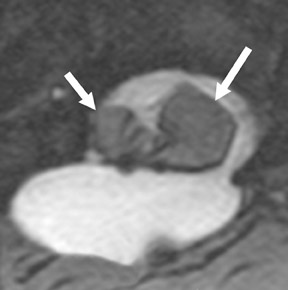
Suboccipital decompression (with or without dural grafting), cyst shunting, and lysis of intradural adhesions is the most commonly performed surgical treatment in the Chiari malformations and associated syringohydromyelia. Suboccipital decompression relieves stenosis at the foramen magnum, which corrects associated flow-related abnormalities and thus prevents syrinx development or enlargement of an existing syrinx. Some researchers advocate intradural exploration to lyse commonly associated intradural adhesions.9 Stents and shunts diverting CSF from the syrinx to the adjacent CSF or into the peritoneal or pleural cavity are favored in cases of isolated syrinx rather than cases associated with Chiari I malformations10 (Figure 3). A multicenter study that evaluated the surgical preferences and outcomes reported that suboccipital decompression was a better treatment option for Chiari I malformations while stents and shunts were more efficacious in treating cases of isolated syrinx.10 Several shunting procedures have been suggested for the correction of symptomatic syringohydromyelia. One multicenter study determined surgical preferences in the following order: syringosubarachnoid shunt, syringopleural shunt, syringoperitoneal shunt, and syringocisternal shunt.11-13
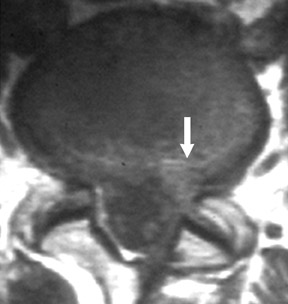
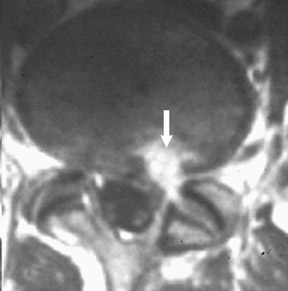
Magnetic susceptibility
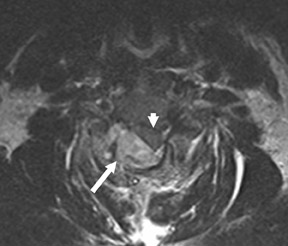
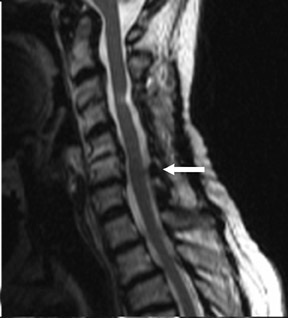
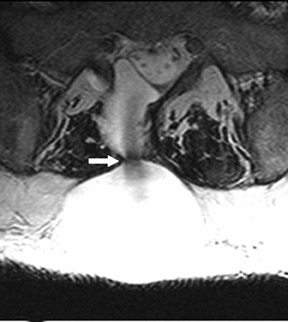
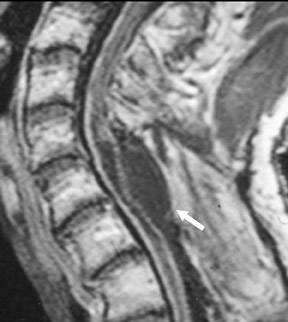
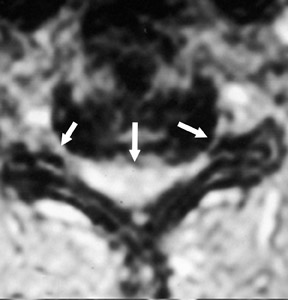
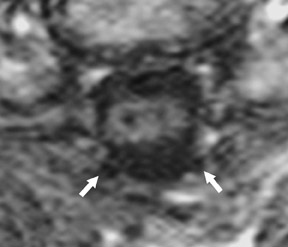
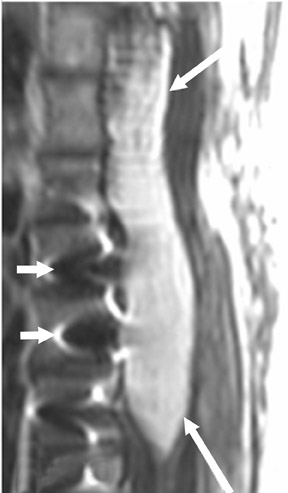
Magnetic susceptibility artifacts are frequently encountered in the postinstrumented spine; particularly when GRE sequences are used. Gradient-recalled echo allows for a significantly decreased acquisition time; however, T2* field inhomogeneities are worsened with GRE because of lack of a refocusing RF pulse.14 Therefore, gradient-recalled echo imaging can be used in certain applications to not only limit acquisition time but also to search for otherwise subtle foci of magnetic susceptibility such as paramagnetic blood products, air/water interfaces, or calcium deposition.14 Magnetic susceptibility due to neurosurgical hardware is increased at higher field strengths and, therefore, the ferromagnetic properties of spinal hardware are amplified as the field strength is increased. The use of fast spin-echo (FSE) T2-weighted (T2W) imaging is preferred in these patients. Phased-array coils with parallel imaging, high bandwidth, and relatively long echo train FSE sequences can be used to further reduce magnetic susceptibility.14 This fact should be taken into special consideration when comparing scans from 1.5T and 3T magnets (Figure 4).15 Artifacts may be significant enough, even with the use of FSE techniques, to limit the evaluation of the spinal canal and the surrounding structures.
Failure of surgical hardware
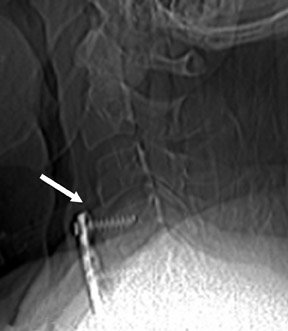
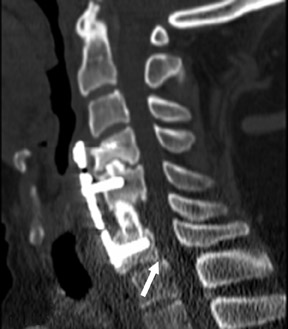
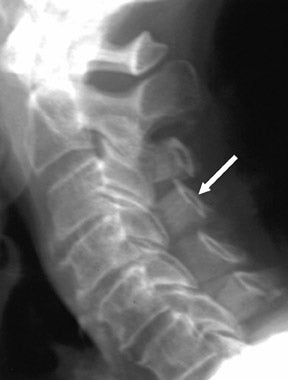
Postoperative spine fracture may occur from osteoporotic insufficiency, neoplasia, subsequent trauma, osteomyelitis, pseudoarthrosis, Charcot joint formation, repetitive stress reaction, hardware failure, or a combination of these entities. Spinal hardware may fracture or migrate from mal-positioning, misconstruction, excessive stress, acute trauma, or metal fatigue.16,17 This potential for hardware compromise requires the radiologist to precisely identify and report the configuration of hardware placement (Figure 5). Transfacet, lateral mass, or transpedicular screws may fracture from metal fatigue, may cause fractures in vertebral body margins, or may enter neural foramen, the spinal canal, adjacent vertebral body levels, or proximate soft tissues.18
Disc versus scar
The removal of herniated discs is one of the most common indications for spinal surgery. Knowledge of the patient’s detailed surgical history is essential, as there may be little evidence of postprocedure changes to indicate any prior operations or interventions. Various terms have been used to describe disc disease in an attempt to improve understanding of the configuration, degree, and location of disc pathology.19-24 Postsurgical findings may include recurrent disc herniation, residual disc material, scar formation, infection, hemorrhage, or a combination of these entities. The hallmark sign distinguishing postoperative scar from recurrent or residual disc herniation is the pattern of enhancement. Scar tissue tends to enhance homogeneously, while disc herniation tends to enhance peripherally (Figure 6). In the early postoperative period (less than 3 to 6 months), it may be impossible to distinguish peripherally enhancing scar type changes from recurrent/residual disc herniation.
Postoperative fluid collections
Postoperative fluid collections are frequent and must be differentiated from seroma, hemorrhage, abscess, and/or pseudomeningocele. Identification is critical because of adjacent vulnerable neural structures and potentially serious sequelae of delayed or inappropriate treatment25–27 Hemorrhage may be identified by observing a blood/serum level on cross-sectional imaging, measuring HU on CT, and/or evaluating the MRI sequence signal characteristics that are indicative of blood products.28 A hemorrhage may be complicated by a superimposed infection, which may be difficult to distinguish from inflammatory scar formation.
Pseudomeningoceles
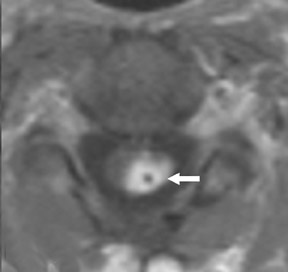
Pseudomeningoceles may enhance peripherally in a manner that may simulate an inflammatory or infectious process (Figure 7). It may be possible to differentiate abscess from pseudomeningocele by observing T1W pre- and postgadolinium and T2W sequences to determine the enhancement characteristics and whether the internal signal exactly follows the signal of CSF.29,30 Pseudomeningoceles do not regularly show the degree of adjacent inflammation and enhancement characteristics that are associated with most abscesses. Pseudomeningoceles exhibit signal that should virtually match CSF intensity. Abscesses, conversely, are expected to display lower signal intensity on T2W sequences and higher signal on T1W sequences because of intermixed proteinaceous products. Infrequently, pseudomeningoceles reveal the site of a leak through the dura and are seen as low signal that represents dephasing of fluid due to flow.
Abscess or phlegmon
A focal region of infectious inflammation, also known as a phlegmon, often precedes frank abscess formation and may enhance with characteristics that are difficult to differentiate from recently formed scar tissue or tumor. An abscess typically presents in an epidural location; however, it may also present in a subcutaneous or intramuscular position or in the intradural space or disc space, and/or it may involve the ligamentum flavum or lie within the cord. An abscess often appears with a thick rind or capsule of inflammatory tissue that enhances avidly.31-33 Spinal epidural abscesses have been shown to occur more commonly in the lumbar spine. In these abscesses, 72% of cultures reveal gram-positive organisms with Staphylococcus aureus as the most common culprit (45%).33 Figure 7F depicts a peripherally enhancing epidural lesion compressing the cord that was culture-positive, consistent with abscess.
Inflammation
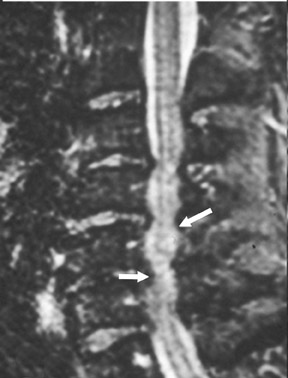
Inflammatory processes such as arachnoiditis, fibrosis, inflammatory pseudotumor, and tethered cord may complicate the postoperative state (Figure 8). MRI evaluation of the lumbar spine following myelomeningocele repair shows findings that are consistent with tethered cord in virtually all patients.34
Spinal canal decompression
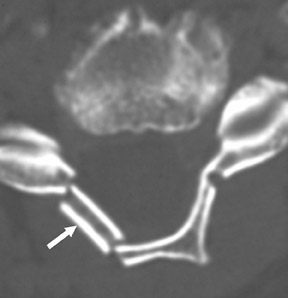
Stenosis of the spinal canal may be congenital or the result of spondylosis, including facet hypertrophy, ligamentum flavum hypertrophy, ossification of the posterior longitudinal ligament, and posterior endplate spurring, which may be exacerbated by intervertebral disc herniation and congenital spinal canal stenosis. Scoliotic deformities, spondylolisthesis, and compressive fractures may also complicate spondylotic stenosis. Spinal stenosis is most common in the cervical and lumbar areas, and the pain associated with neural compression may be ameliorated by decompressive surgical procedures such as partial disc resection, laminectomy, unilateral laminotomy, bilateral laminotomy, laminoplasty, and other surgical variations.35,36 Laminoplasty of the cervical spine has become an accepted procedure for alleviating focal or multilevel spinal canal stenosis (Figure 9). Hydroxyapatite, ceramics, and other material have been used to promote structural stability. Osseous autografts, allografts, and xenografts (including the fibula, iliac crest, vertebral bodies, and other sources) have been used extensively for spinal reconstruction.37-39
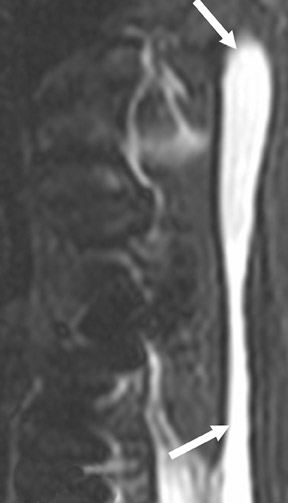
Dural autografts
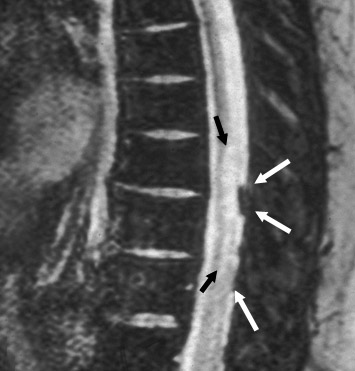
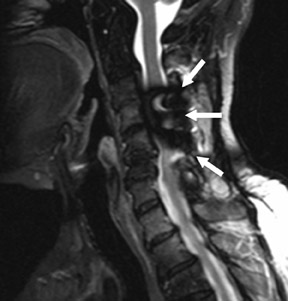
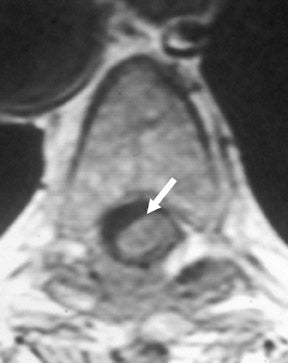
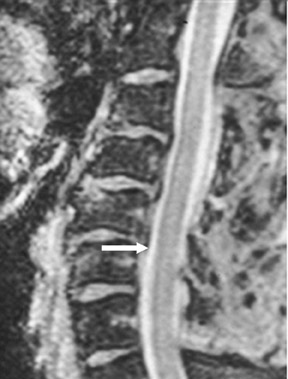
Dural grafts are used for the repair of some congenital malformations, following dural resection, or in cases requiring enlargement of the dural sac. It is important to recognize the expected presentation of a dural graft (Figure 10) and to differentiate it from other potentially associated complications, such as scar tissue, complicated pseudomeningocele, infection, or CSF leak.40 It is essential that the radiologist is familiar with the patient’s surgical history, since this presentation may be inadvertently interpreted as an inflammatory or infectious process.
Complicated postoperative spine
A complicated postoperative spine may include multiple surgical hardware revisions, hardware compromise, complicated fluid collections, tumor recurrence, subsequent osteoporotic insufficiency fractures, a mixture of the above, or other complications. Figure 11 displays one example of the plethora of potential complicated postoperative spine presentations.
Conclusion
Interpreting postoperative spine imaging requires a systematic approach and knowledge of the patients’ surgical history. This approach will allow the radiologist to separate the many possible confounding findings. Obtaining surgical reports and detailed clinical history can be invaluable in understanding and properly reporting on the postoperative spine.
Acknowledgement
The authors would like to thank Jennifer I. Hui for her assistance with the preparation of this manuscript.
REFERENCES
- Goodrich JT. History of spine surgery in the ancient and medieval worlds. Neurosurg Focus. 2004;16:E2.
- Slone RM, MacMillan M, Montgomery WJ. Spinal fixation. Part 1. Principles, basic hardware, and fixation techniques for the cervical spine. RadioGraphics. 1993;13:341-356.
- McDonnell DE. History of spinal surgery: One neurosurgeon’s perspective. Neurosurg Focus. 2004;16:E1.
- Taber KH, Herrick RC, Weathers SW, et al. Pitfalls and artifacts encountered in clinical MR imaging of the spine. RadioGraphics. 1998;18:1499-1521.
- Pospiech J, Kalff R, Pajonk F, et al. The scar tissue protective effect of free autologous fatty tissue transplants. an animal experiment on spinal surgery [in German]. Langenbecks Arch Chir. 1994; 379(3):137-144.
- Krishnamoorthy T, Thomas B. Unknown case. Spine. 2006;31:1633-1634.
- Haughton VM, Ho KC. Effect of blood on arachnoiditis from aqueous myelographic contrast media. AJR Am J Roentgenol. 1982;139:569-570.
- Teplick JG, Haskin ME. Intravenous contrast-enhanced CT of the postoperative lumbar spine: Improved identification of recurrent disk herniation, scar, arachnoiditis, and diskitis. AJR Am J Roentgenol. 1984;143:845-855.
- Holly LT, Johnson JP, Masciopinto JE, Batzdorf U. Treatment of posttraumatic syringomyelia with extradural decompressive surgery. Neurosurg Focus. 2000;8(3):E8.
- Wisoff JH, Epstein F. Management of hydromyelia. Neurosurgery. 1989;25:562-571.
- Haroun RI, Guarnieri M, Meadow JJ, et al. Current opinions for the treatment of syringomyelia and chiari malformations: Survey of the Pediatric Section of the American Association of Neurological Surgeons. Pediatr Neurosurg. 2000;33:311-317.
- Greitz D. Unraveling the riddle of syringomyelia. Neurosurg Rev. 2006;29:251-263; discussion 264.
- Pinna G, Alessandrini F, Alfieri A, et al. Cerebrospinal fluid flow dynamics study in Chiari I malformation: Implications for syrinx formation. Neurosurg Focus. 2000;8:E3.
- Cardoza JD, Herfkens RJ.MRI Survival Guide. 1st ed. New York, NY: Raven Press; 1994.
- Allkemper T, Tombach B, Schwindt W, et al. Acute and subacute intracerebral hemorrhages: Comparison of MR imaging at 1.5 and 3.0 T–Initial experience. Radiology. 2004;232:874-881.
- Berquist TH. Imaging of the postoperative spine. Radiol Clin North Am. 2006;44:407-418.
- Gerscovich EO, Greenspan A, Montesano PX. Treatment of kyphotic deformity in ankylosing spondylitis. Orthopedics. 1994;17:335-342.
- Hunter TB, Yoshino MT, Dzioba RB, et al. Medical devices of the head, neck, and spine. RadioGraphics. 2004;24:257-285.
- Fardon DF, Milette PC, Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine. 2001;26(5):E93-E113.
- Wiltse LL, Berger PE, McCulloch JA. A system for reporting the size and location of lesions in the spine. Spine. 1997;22:1534-1537.
- Appel B. Nomenclature and classification of lumbar disc pathology. Neuroradiology. 2001;43:1124-1125.
- Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873-1878.
- Fardon DF. Nomenclature and classification of lumbar disc pathology. Spine. 2001;26:461-462.
- Wiltse LL, Berger PE, McCulloch JA. A system for reporting the size and location of lesions in the spine. Spine. 1997;22:1534-1537.
- Chen HC, Hsu PW, Lin CY, Tzaan WC. Symptomatic hematoma of cervical ligamentum flavum: Case report. Spine. 2005;30:E489-E491.
- Croisille P, Vandermarcq P, Ferrie JC, et al. MRI in infectious and spontaneous tumoral epidural hemorrhagic pathology [in French]. J Radiol. 1993;74:399-407.
- Runge VM, Williams NM, Lee C, Timoney JF. Magnetic resonance imaging in a spinal abscess model. Preliminary report. Invest Radiol. 1998;33:246-255.
- Quencer RM, Montalvo BM, Eismont FJ, Green BA. Intraoperative spinal sonography in thoracic and lumbar fractures: Evaluation of Harrington rod instrumentation. AJR Am J Roentgenol. 1985;145:343-349.
- Sarrazin JL. Imaging of postoperative lumbar spine. J Radiol. 2003;84(2 Pt 2):241-250;quiz 251-252.
- Ross JS. Magnetic resonance imaging of the postoperative spine. Semin Musculoskelet Radiol. 2000;4:281-291.
- Bluman EM, Palumbo MA, Lucas PR. Spinal epidural abscess in adults. J Am Acad Orthop Surg. 2004;12:155-163.
- Tay BK, Deckey J, Hu SS. Spinal infections. J Am Acad Orthop Surg. 2002;10:188-197.
- Del Curling O Jr, Gower DJ, McWhorter JM. Changing concepts in spinal epidural abscess: A report of 29 cases. Neurosurgery. 1990;27:185-192.
- Hudgins RJ, Gilreath CL. Tethered spinal cord following repair of myelomeningocele. Neurosurg Focus. 2004;16(2):E7.
- Kostuik JP. Medicolegal consequences of cauda equina syndrome: An overview. Neurosurg Focus. 2004;16(6):39-41.
- Hansraj KK, Cammisa FP Jr, O’Leary PF, et al. Decompressive surgery for typical lumbar spinal stenosis. Clin Orthop Relat Res. 2001;384:10-17.
- Xie Y, Chopin D, Hardouin P, Lu J. Clinical, radiological and histological study of the failure of cervical interbody fusions with bone substitutes. Eur Spine J. 2006;15:1196-1203.
- Schultheiss M, Sarkar M, Arand M, et al. Solvent-preserved, bovine cancellous bone blocks used for reconstruction of thoracolumbar fractures in minimally invasive spinal surgery-first clinical results. Eur Spine J. 2005;14:192-196.
- Malca SA, Roche PH, Rosset E, Pellet W. Cervical interbody xenograft with plate fixation: Evaluation of fusion after 7 years of use in post-traumatic discoligamentous instability. Spine. 1996;21:685-690.
- Martínez-Lage JF, Pérez-Espejo MA, Palazón JH, et al. Autologous tissues for dural grafting in children: A report of 56 cases. Childs Nerv Syst. 2006;22:139-144.
Related Articles
Citation
. Challenges and pitfalls in postoperative spine imaging. Appl Radiol.
October 11, 2011