Breast masses in children and adolescents
Images






In the pediatric and adolescent population, breast masses are nearly always benign, self-limited and managed conservatively, in contrast to adults, where malignant tumors have a relatively high incidence and often require excision. 1-5 In addition, normal developmental variants, which can mimic tumor, are more common in a young population. This article describes the imaging features of developmental variants and breast masses in children and adolescents, and the associated clinical findings that can facilitate the diagnosis and appropriate treatment of these patients.
Imaging evaluation of the pediatric breast
Ultrasonography is the mainstay of breast imaging in children and adolescents, in contrast to adults, where mammography is the standard screening study.1,2,5 Mammography is not used routinely in a young population since the pediatric breast is denser than the adult breast, which can limit the sensitivity of mammography. Mammography also utilizes ionizing radiation, and the principle of radiation exposure in a young population is to keep the radiation dose as low as reasonably achievable. Computed tomography and magnetic resonance imaging (MRI) are ancillary studies that can be helpful in characterizing breast masses when sonography is equivocal and in assessing the extent of metastatic disease or a primary tumor for surgical planning.
Normal breast development
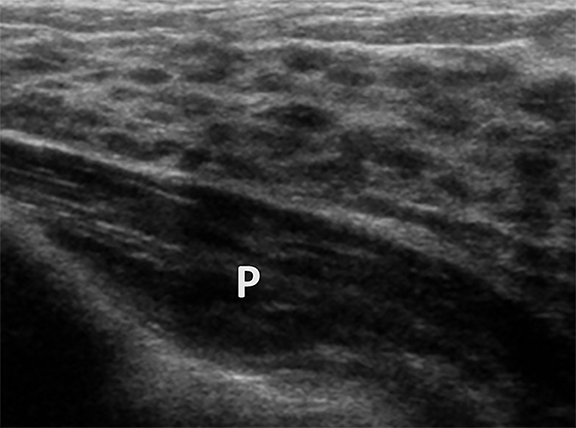
Ultrasound images prior to thelarche show a band of echogenic retroareolar tissue anterior to the pectoralis muscle (Figure 1A). When breast development starts, the breast bud appears as a retroareolar hypoechoic mass (Figure 1B). As breast development continues the fibrofatty and glandular tissues increase. In the mature breast, sonography shows the anterior subcutaneous fat layer, subadjacent glandular tissue, which is echogenic, and fat which is hypoechoic, and posterior pectoralis muscle. Linear echogenic band representing connective tissue may be noted within the fatty tissue (Figure 1C).
Developmental breast masses
Premature thelarche
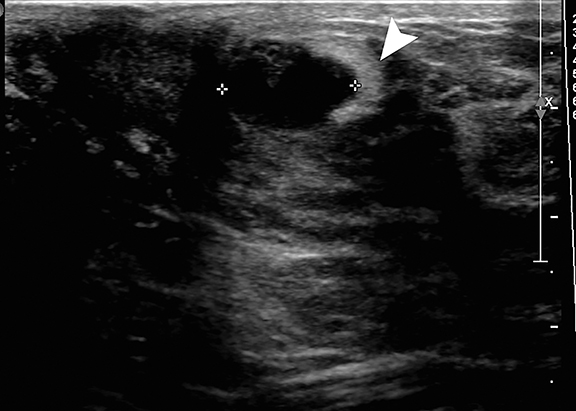
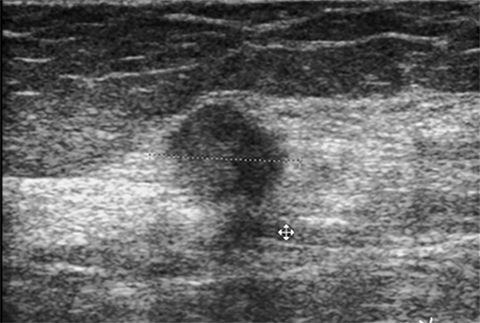
Premature thelarche is the onset of female breast development prior to the age of 8 yrs. It may be isolated or associated with true precocious puberty. Isolated premature thelarche usually occurs in girls between 1 and 3 years of age, who present with unilateral or bilateral palpable subareolar masses. Sonography shows a hypoechoic mass in the subcutaneous tissues anterior to the chest wall musculature, representing a developing breast bud (Figure 2).
Gynecomastia
Gynecomastia is enlargement of the male breast, which clinically presents as a tender, firm subareolar nodule. Physiologic gynecomastia has peak frequencies at two times: in the neonatal period and during adolescence. In neonates, breast enlargement is related to maternal hormone stimulation in utero.5 During puberty, gynecomastia occurs at about age 13-14 years and as many as 75% of male adolescents are affected. It typically resolves within several months to 2 years.4,6 Pathologic gynecomastia is associated with feminizing adrenocortical tumors, estrogen-producing testicular tumors (Sertoli or Leydig cell), gonadotropin-secreting tumors and other conditions associated with elevated gonadotropin levels (hepatoblastoma, prolactinomas, Klinefelter syndrome, testicular feminization syndrome, and neurofibromatosis type 1 and drugs (estrogens, anabolic steroids, digitalis, corticosteroids, tricyclic antidepressants and marijuana).4,6 Regardless of cause, sonography shows hypoechoic breast tissue in the subareolar area, similar to that seen in early stages of normal breast development in female adolescents and in premature thelarche (Figure 1B and Figure 2).1,2,5,6
Non-neoplastic cystic lesions
Ductal ectasia
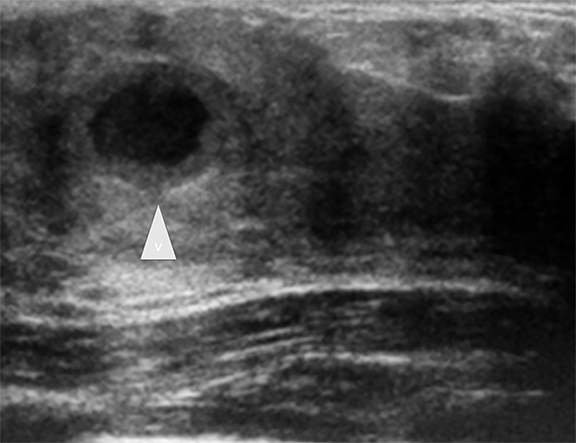
Duct ectasia occurs when a retroareolar duct fills with fluid. The condition often causes no symptoms, but some adolescents may present with a palpable mass, nipple discharge, breast tenderness or inflammation (periductal mastitis).1-4 Ultrasonography shows retroareolar tubular anechoic structures, representing the fluid-filled ducts. Infected ducts may contain debris or septae (Figure 3).1-3,7
Fibrocystic disease
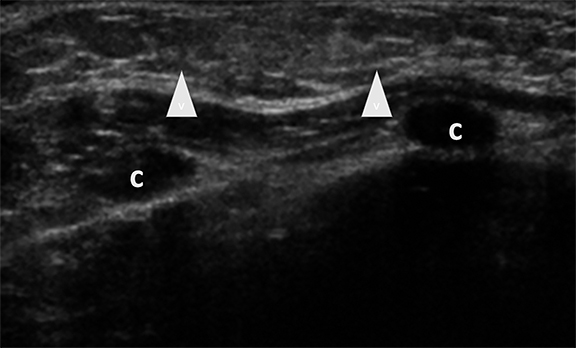
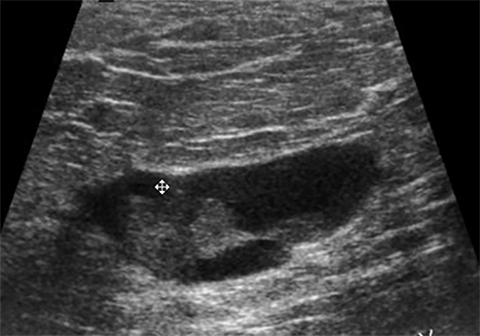
Affected adolescent females present with cyclically tender breasts that are nodular on palpation.1-3 Sonographic findings include single or multiple cysts, dilated ducts and highly echogenic foci with posterior acoustic shadowing, representing fibrous tissue (Figure 4).1,2 These cysts may be simple or complex and vary widely in size.
Posttraumatic lesions
Posttraumatic hematomas and fat necrosis are most often secondary to sports-related injuries, presenting as tender masses.4 On ultrasound, the acute hematoma is hyperechoic to surrounding tissues, becoming complex with internal echoes and eventually anechoic over time (Figure 5). Fat necrosis appears as an echogenic, complex mass with cystic spaces or as a hypoechoic mass with or without posterior acoustic shadowing.
Mastitis and abscess
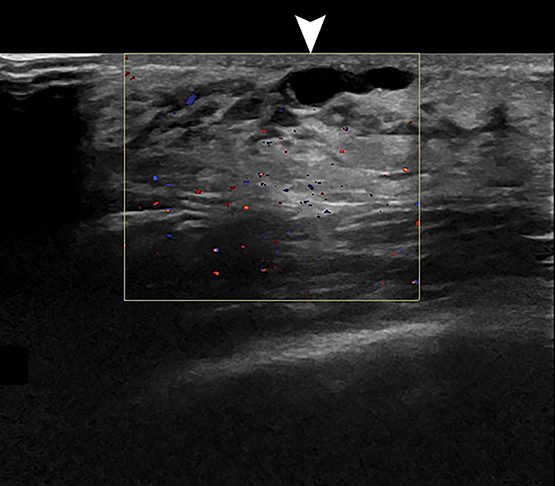
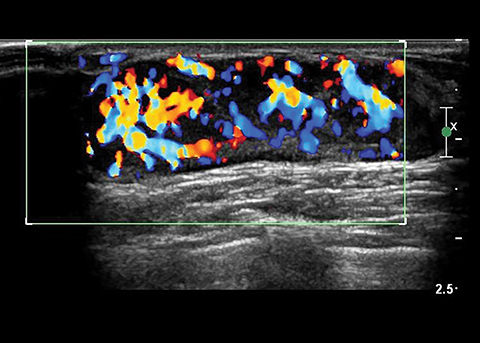
Inflammatory lesions can result from infection of an obstructed subareolar duct, chest wall area cellulitis, and nipple piercing or breast trauma.1,2,4,8,9 Affected patients present with a tender, indurated erythematous breast. At sonography, mastitis appears as thickened, echogenic breast tissue with central color Doppler flow. An abscess appears as a hypoechoic, thick-walled mass with internal debris and peripheral color Doppler flow (Figure 6).
Benign neoplastic masses
Fibroadenoma
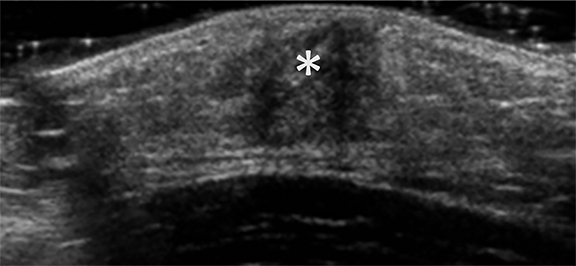
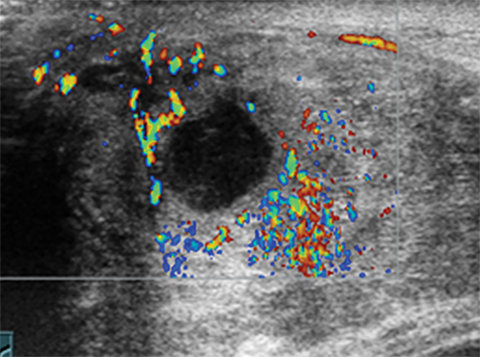
Fibroadenomas are the most common solid breast masses in this population, comprising up to 90% of breast masses.1,2,10 Classically, fibroadenomas are mobile non-tender breast masses. The characteristic sonographic features are a well-circumscribed hypoechoic mass that that is oval in shape with the width greater than the height. The diameter usually ranges from 2 to 3 cm. Color Doppler commonly shows internal flow (Figure 7). 1,2,5,9,10 The term giant fibroadenoma is reserved for fibroadenomas that are greater than 5 cm in diameter. The giant fibroadenoma may contain fluid-filled clefts (Figure 8).9,10
Phyllodes tumors, also known as cystosarcoma phyllodes, are histologically similar to fibroadenomas but show more stromal proliferation. Affected patients present with a large, painless firm, mobile mass.3 Phyllodes tumors may be benign, intermediate, or malignant. The malignant tumors have increased mitotic rate and show similarity to sarcomas.11 Despite their histologic classification, all phyllodes tumors have potential to metastasize and recur locally. Sonographic findings are similar to the giant fibroadenoma (Figure 9).1,2,5,12 Differentiation requires tissue sampling.
Hemangioma
Infantile hemangioma is a common breast mass in the young infant. It undergoes a phase of initial growth, followed by involution over months to several years and thus is managed conservatively. Hemangioma is hypoechoic relative to surrounding tissue and shows vascular channels on color Doppler images (Figure 10).2
Juvenile papillomatosis
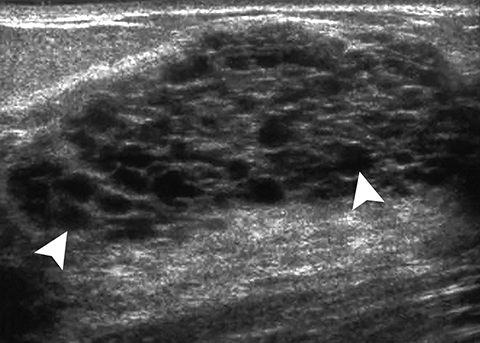
Juvenile papillomatosis, also termed “Swiss cheese” disease, typically presents as a firm, mobile mass in the periphery of the breast in adolescents and young women.3 It has been associated with a positive family history of breast cancer (33% to 58% of cases) and about 5% to 15% of affected patients have concurrent breast cancer.1 At sonography, it appears as a complex mass with small cysts and echogenic septations and ill- or well-defined margins (Figure 11).1-3,13
Intraductal papilloma
Intraductal papilloma is a benign proliferation of ductal epithelium that can occur in adolescent girls and boys.1,2,14 Patients often present with serous or serosanguinous nipple discharge. Sonographic findings are an echogenic intraductal mass with or without ductal dilatation and with internal flow on Doppler imaging (Figure 12).14
Pseudoangiomatous stromal hyperplasia
Pseudoangiomatous stromal hyperplasia (PASH) is a benign myofibroblastic proliferation.1,2,5 Affected patients present with a painless, firm, mobile mass. Sonographic findings are a well-circumscribed, hypoechoic, ovoid mass with the long axis oriented parallel to the chest wall, similar to the fibroadenoma (Figure 13). 1,2,5,15 Differentiation from fibroadenoma requires tissue sampling.
Breast masses from metastatic disease and primary breast cancer
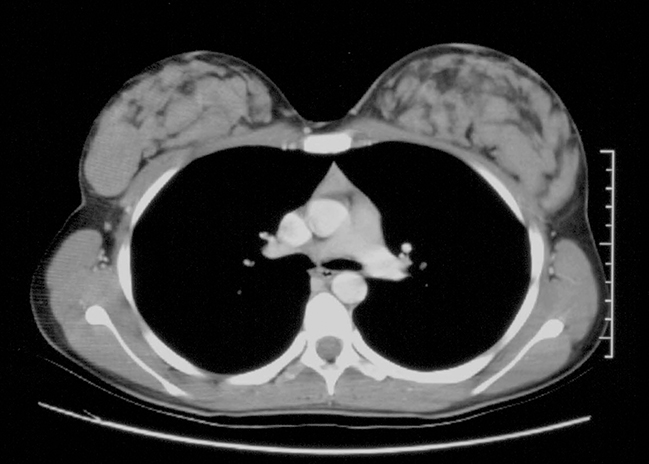
Malignant tumors in the pediatric age group are extremely rare, accounting for less than 5% of breast masses, and they are more likely to be metastatic than a primary breast cancer.1-3,11 Primary malignancies that metastasize to the breast include rhabdomyosarcoma, neuroblastoma, renal carcinoma, Hodgkin’s lymphoma, and leukemia.1,2,16 Radiation exposure during breast development is a risk factor to development of breast cancer later in life.17 Secretory carcinoma is the most common primary breast cancer followed by intraductal cancer.18 Medullary and inflammatory cancers have also been described in pediatric patients, although these are far less common than secretory carcinoma.11
On physical examination, primary breast cancer and metastases present as a hard, irregular shaped, non-mobile mass. At sonography, both masses appear as hypoechoic lesions with irregular, spiculated or lobulated margins, internal heterogeneity, a long axis perpendicular to the chest wall and variable posterior acoustic shadowing (Figures 14,15).1,2,16,19
Conclusion
Pediatric breast masses are relatively rare in children and adolescents, and the spectrum is different from that in adults. Most lesions are benign, either variations in normal breast development or a neoplastic process with a benign behavior. Fortunately, primary breast cancer and metastatic lesions are incredibly rare. Because of its lack of radiation, ultrasonography is the ideal imaging study to identify, characterize and guide treatment of breast lesions in a young population. Awareness of the ultrasound appearance of normal breast development and specific lesions along with the clinical findings is essential to enable an accurate diagnosis.
References
- Chang E, Cube R, Hall GJ, et al. Breast masses in children and adolescents: Radiologic-pathologic correlation. Radiographics. 2009; 29:907-931.
- Chung E, Siegel MJ. Breast. In Siegel MJ, ed. Pediatric Sonography. 4th ed. Philadelphia, PA :Lippincott, Williams and Wilkins; 2010: 200-213
- DeSilva NK, Brandt ML. Disorders of the breast in children and adolescents, Part 2: Breast masses. J Pediatr Adolesc Gynecol. 2006; 19:415-418.
- Greydanus DE, Matytsina L, Gains M. Breast disorders in children and adolescents. Prim Care. 2006; 33:455-502.
- Kaneda HJ, Mack J, Kasales CJ, et al. Pediatric and adolescent breast masses: a review of pathophysiology, imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2013; 200:W204-W212.
- Welch ST, Babcock DS, Ballard ET. Sonography of pediatric male breast masses: gynecomastia and beyond. Pediatr Radiol. 2004; 34:952-957.
- Huneeus A, Schilling A, Horvath E, et al. Retroareolar cysts in the adolescent. J Pediatr Adolesc. Gynecol. 2003; 16:45-49.
- Borders H, Mychaliska G, Gerbarski KS. Sonographic features of neonatal mastitis and breast abscess. Pediatr Radiol. 2009; 39:955-58.
- Kronemer KA, Rhee K, Siegel MJ, et al. Gray scale sonography of breast masses in adolescent girls. J Ultrasound Med. 2001; 20:491-496.
- Sanchez R, Ladino-Torres MF, Bernat JA, et al. Breast fibroadenomas in the pediatric population: common and uncommon sonographic findings. Pediatr Radiol. 2010; 40:1681-1689.
- Kennedy RD, Boughedy JC. Management of pediatric and adolescent breast masses. Semin Plast Surg. 2013; 27:19-22.
- Yilmaz E, Sal S, Lebe B. Differentiation of phyllodes tumors versus fibroadenomas. Acta Radiol. 2002; 43:34-39.
- Kersschot EA, Hermans ME, Pauwels C, et al. Juvenile papillomatosis of the breast: sonographic appearance. Radiology. 1988; 169:631-633.
- Ganesan S, Karthik G, Joshi M, et al. Ultrasound spectrum in intraductal papillary neoplasms of breast. Br J Radiol. 2006; 79:843-849.
- Mercado CL, Naidrich SA, Hamele-Bena D, et al. Pseudoangiomatous stromal hyperplasia of the breast: sonographic features with histopathologic correlation. Breast J. 2004; 10:427–432.
- Kumar PRG, Grossman Z, Scorza L, et al. Isolated extramedullary relapse of acute lymphoblastic leukemia in the breast of an adolescent girl: radiologic findings and discussion. Pediatr Radiol. 2010; 40:773-776.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014; 32:2217-2223.
- Horowitz DP, Sharma CS, Connolly E, et al. Secretory carcinoma of the breast: results from the survival, epidemiology and end results database. Breast. 2012; 21:350–353.
- Mun SH, Ko EY, Han BK, et al. Secretory carcinoma of the breast: sonographic features. J Ultrasound Med. 2008; 27:947–954.
Citation
MJ S, E C.Breast masses in children and adolescents. Appl Radiol. 2017; (9):12-17.
September 7, 2017