Bisphosphonate associated osteonecrosis of the jaw
Images
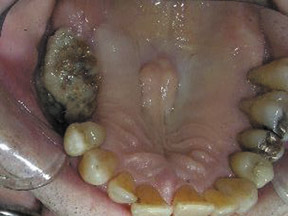
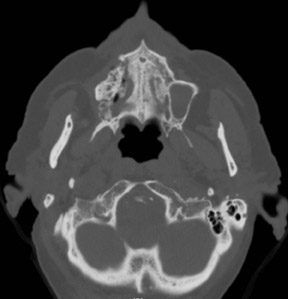


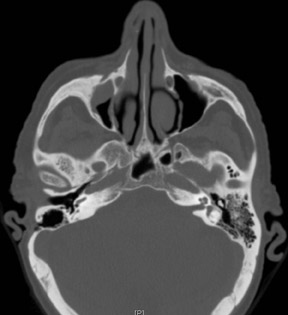
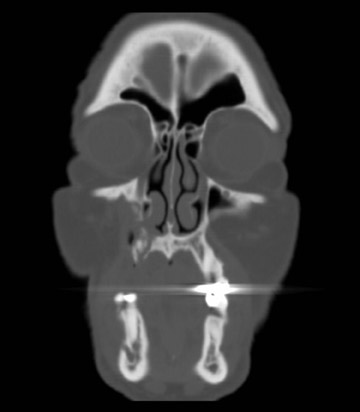
Bisphosphonate associated osteonecrosis of the jaw
Findings
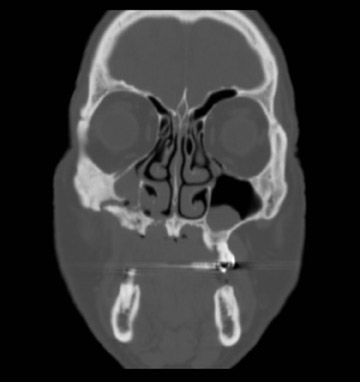
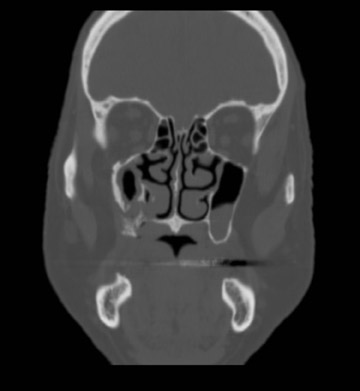
Axial and coronal computed tomography (CT) images of the maxillofacial bones (Figures 2 and 3) demonstrate extensive cortical destruction, osteosclerosis, cortical thickening, and fragmentation of the right maxilla, which extends into the frontal process of the zygomatic bone and pterygoid plates. The anterior wall of the maxillary sinus, as well as the palatine process, are mildly depressed, and the lateral and medial walls of the sinus are fragmented, with several bony sequestra present within the sinus cavity. There is sparing of the orbital floor. There is an oroantral fistula, mucosal thickening of the maxillary sinus, and a small amount of soft tissue gas extending from the fragmented sinus. A small amount of soft tissue fluid is noted within the right cheek; however, there is no evidence of soft tissue infection. An incidental note was made of a left maxillary sinus mucus retention cyst, with an otherwise spared contralateral maxilla. The mandible (not shown) was uninvolved.
Discussion
Bisphosphonate associated osteonecrosis of the jaw (BON) is defined as the unexpected development of necrotic bone in the oral cavity of a patient who is receiving bisphosphonate treatment and has not received radiotherapy to the head and neck. BON is an evolving complication whose incidence has steadily increased since the clinical use of bisphosphonates in oncology was first approved and their intravenous use in treating patients with bone metastasis was introduced in 1995.1 The first bisphosphonate treatment of patients with osteonecrosis of the jaw (ONJ) was reported in 1997.2 Since then, more than 2,000 cases have been reported to the United States Food and Drug Administration (FDA).3 Although long familiar to those in oral and maxillofacial surgery and dental circles, BON was not yet well known in radiology at the time of this writing. However, its recognition is essential to patient management, as timely diagnosis can impact patient morbidity considerably.
The bisphosphonates, which are non-metabolized synthetic analogs of pyrophosphate synthetase, function through their inhibition of osseous resorption via suppression of osteoclast activity. The use and efficacy of these agents (intravenously administered pamidronate, zoledronate, ibandronate), in concurrence with antineoplastic chemotherapy, has been well documented in the treatment of bone pain, moderate to severe hypercalcemia, and other skeletal complications associated with such malignancies as metastatic breast cancer and multiple myeloma. Indeed, the indications for these agents have more recently been extended to include osteolytic lesions arising from any solid tumor (e.g., prostate and lung). Additional uses include osteoporosis (orally administered alendronate and risedronate), Paget’s disease, osteogenesis imperfecta, and fibrous dysplasia.2,4,5
The incidence of BON has been reported to range from 2% to 12% of individuals treated with nitrogen containing bisphosphonates — most commonly, those patients being treated for multiple myeloma.6–9 In a systematic review of 368 published cases of BON by Woo et al., a prevalence of 6% to 10% was estimated in patients undergoing treatment for malignancy with bisphosphonates. This review also found that 94% of cases occurred in patients who were receiving intravenous bisphosphonate therapy for multiple myeloma and metastatic disease to the skeleton.6,8,9 This likely reflects a combination of the higher incidence of involvement of the mandible and maxilla in multiple myeloma relative to that of breast cancer or other solid tumor osseous metastasis, and the tendency of bisphosphonates to accumulate in regions of high bone turnover. Dimopoulos et al., in an analysis of 202 patients undergoing bisphosphonate therapy for multiple myeloma, demonstrated that the particular bisphosphonate being utilized and the mean duration of exposure play a statistically significant role in the development of ONJ. Of the 15 patients who developed BON in this series, only 1 was undergoing therapy with pamidronate alone, whereas the remainder were undergoing therapy with zoledronate alone, or zoledronate in combination with pamidronate or ibandronate. The median time between exposure and onset was 39 months in those who developed osteonecrosis, a time which was shown to be more than halved when zoledronic acid was used alone.10 The initial onset of oral lesions following bisphosphonate therapy has, however, been shown to manifest as early as 46 months.2,5,9 The intravenous use of compounds containing an aminoterminal group (e.g., pamidronate) or a nitrogen containing side chain (e.g., zoledronic acid) has been reported to pose the highest risk; however, this condition has also been reported, albeit less frequently, in individuals undertaking oral bisphosphonate therapy for osteoporosis.1
Bisphosphonates, which bind avidly to exposed bone mineral around resorbing osteoclasts with subsequent internalization by the osteoclasts,5 have demonstrated their efficacy through a number of mechanisms to include induction of osteoclast apoptosis, alterations in enzymatic function of osteoclasts, and incorporation of the molecule into the hydroxyapatite matrix, with resultant modification in bone micro-architecture.3 More recently, these compounds have additionally shown potent anti-angiogenic properties via their ability to appreciably reduce circulating levels of vascular endothelial growth factor (VEGF), with inhibition of bone blood flow ultimately responsible for bone resorption and loss, creating an ideal milieu for the subsequent development of osteonecrosis.3,4,11 Direct apoptosis of tumor cells, adhesional inhibition of malignant cells to, and over, bone matrix in vitro, and inhibition of various metalloproteinases involved in cancer growth and metastasis in vitro have also been shown.1,3,4,11
Presenting symptoms have been reported relatively consistently throughout the literature, with patients typically complaining of a painful, “nonhealing” extraction socket or exposed bone with progression to sequestrum formation associated with localized swelling and purulent discharge.4,5 Although surgical extractions/jaw trauma and radiotherapy have commonly been identified as predisposing factors for osteonecrosis, there are reports of spontaneous exposures and necrosis of the alveolar bone,12 as shown by Ruggiero et al., where 9 of 63 patients (14%) had no history of recent dentoalveolar procedures, yet presented with spontaneous bony exposure and alveolar bone necrosis.
BON may remain asymptomatic for weeks or months; it is often recognized solely by exposed bone in the oral cavity. Patients may become symptomatic following a secondary infection or trauma to adjacent and/or opposing normal soft tissues, from the irregular surfaces created by the exposed bone. Unfortunately, a diagnosis is usually not established until the disease is well advanced, as patients usually do not present until osteonecrosis has already become symptomatic.4
Because the body does not metabolize bisphosphonates, which have a bone half-life ranging from several months to years and accumulate specifically in active bony sites with increased osteoclastic activity, it has been postulated that the skeleton acts as a reservoir where high concentrations of the compounds are maintained for extended periods of time. This could explain why osteonecrosis appears after long-term treatment and why it persists, and even progresses, in cases where bisphosphonate treatment has been discontinued.5,6,8,11,12
What, then, explains the predilection of this condition for the jaw and its greater incidence in those who have experienced previous surgical extractions and/or jaw trauma? Several theories suggest ONJ likely results from a combination of the induction of osteoclast and oral mucosa keratinocyte apoptosis, and the inhibitory effects upon VEGF. This combination, the thinking goes, results in the inability of hypodynamic and hypovascular bone to meet increased demand for repair and remodeling, owing to the innate, continual physiologic stress (mastication), iatrogenic trauma (tooth extraction or denture injury), or dental infection within an environment laden with oral flora bacteria.13,12 The chemotherapeutic agents and steroid preparations taken by these patients also influence wound healing and, therefore, necessitate consideration as possible etiologic factors.1,5
In the vast majority of literature reviewed at the time of this writing, disease involvement was consistently more prevalent in the mandible than the maxilla (hence, the alternative terminology, “bisphosphonate associated osteonecrosis of the mandible”). Involvement of the maxilla, however, is not infrequent despite its collateral circulation and rich vascular supply. Ruggerio et al. demonstrated that, in contrast with patients who typically develop osteoradionecrosis of the jaw, maxilla involvement was common in bisphosphonate therapy, representing 24 of 63 patients (38%) in their sample population.5 Migliorati et al. even suggest that, in BON, the maxilla is affected much more commonly than the mandible.9
A recent study by Chiandussi et al. employed conventional radiography, CT, magnetic resonance imaging (MRI), and a 99-Tcm-MDP3-phase bone scan on each of 11 individuals with BON in an effort to describe the features of each modality and aid the prompt recognition of the condition.
The investigators concluded that although panoramic plain radiography offers good specificity in distinguishing osteonecrosis from other pathologies, and is further exceeded by both CT and MRI, none of the modalities is able to differentiate bisphosphonate induced osteonecrosis of the jaw from other well established causes of exposed bone in the jaw, such as osteoradionecrosis (which also shares strikingly similar clinical features with BON), osteonecrosis secondary to osteomyelitis, or steroid induced osteonecrosis (the latter being an uncommon occurrence).4,5 CT, including multiplanar reformatting (MPR), as was undertaken in this particular case, has shown its utility in defining the features and extent of both osseous and soft tissue involvement of this process. In select circumstances, MRI has been able to further augment diagnosis by establishing the degree of soft tissue involvement. Scintigraphy has also been shown to be a very sensitive modality; therefore, it has been suggested as a potential screening tool to detect subclinical disease in patients undergoing bisphosphonate therapy.1,4
Conclusion
Bisphosphonate associated osteonecrosis of the jaw represents a serious side effect of bisphosphonate administration. Although treatment regimens, including discontinuation of the offending drug, surgical debridement/sequestrectomy, hyperbaric oxygen, and longterm antibiotic therapy have been advocated, a universally accepted, standardized treatment protocol for BON had not yet been adopted at the time of this writing, as none of these treatments alone, or in combination, had proven consistently efficacious. Consequently, the focus rests on prevention and early detection, as timely diagnosis can influence disease outcome considerably.
- Migliorati CA, Siegel MA, Elting LS. Bisphosphonateassociated osteonecrosis: a longterm complication of bisphosphonate treatment. Lancet Oncol. 2006;7:508-514.
- Dimitrakopoulos I, Magopoulos C, Karakasis D. Bisphosphonateinduced avascular osteonecrosis of the jaws: a clinical report of 11 cases. Int J Oral Maxillofac Surg. 2006;35:588-593.
- Assael LA. A Time for Perspective on Bisphosphonates. J Oral Maxillofac Surg. 2006;64:877-879.
- Chiandussi S, Biasotto M, Dore F et al. Clinical and diagnostic imaging of bisphosphonateassociated osteonecrosis of the jaws. Dentomaxillofac Radiol. 2006;35:236-243.
- Ruggiero SL, Mehrotra B, Rosenberg TJ et al. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527-534.
- Ashcroft J. Bisphosphonates and phossyjaw: breathing new life into an old problem. Lancet Oncol. 2006;7:447-449.
- Bamias A, Kastritis E, Bamia C et al. Osteonecrosis of the Jaw in Cancer After Treatment With Bisphosphonates: Incidence and Risk Factors. J Clin Oncol. 2005;23:8580-8587.
- Delibasi T, Altundag K, Kanlioglu Y. Why osteonecrosis of the jaw after bisphosphonates treatment is more frequent in multiple myeloma than in solid tumors. J Oral Maxillofac Surg. 2006;64:995-996.
- Woo SB, Hellstein JW, Kalmar JR. Systematic Review: Bisphosphonates and Osteonecrosis of the Jaws. Ann Intern Med. 2006;144:753-761.
- Dimopoulos MA, Kastritis E, Anagnostopoulos A et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica 2006; 91:968971. 2006;91:968-971.
- Merigo E, Manfredi M, Meleti M et al. Jaw bone necrosis without previous dental extractions associated with the use of bisphosphonates (pamidronate and zoledronate): a fourcase report. J Oral Pathol Med. 2005; 34:613-617.
- Leite AF, Figueiredo PT, Melo NS et al. Bisphosphonateassociated osteonecrosis of the jaws. Report of a case and literature review. Oral Surg Oral Pathol Oral Radiol Endod. 2006;102:1421.