Beyond splenomegaly: An image-based review of infectious and inflammatory diseases of the spleen
Images






In abdominal imaging, signs of infectious and inflammatory disease may present solely in the spleen. Examples include bacterial, viral, fungal and mycobacterial infections which may present with discrete splenic findings: mononucleosis, Bartonella, malaria, tuberculosis, and Pneumocystis. Special consideration is given to potential bioterrorism agents, Burkholderia species and Brucella. Finally, autoimmune disease, sarcoidosis, and sarcoid-like reaction are examples of inflammatory disease which may have splenic manifestations.
Splenomegaly
As the spleen is predominantly composed of water, spleen volume and spleen weight, the measurement used by pathologists, can be used interchangeably. Early computed tomography (CT) studies derived formulae to estimate spleen volume from three linear perpendicular measurements, although these differ from the ellipsoid volume calculation often used in sonography.1
There is no agreement on what constitutes ‘normal’ spleen volume. Pathologists have recognized that spleen weight does not follow a bell-shaped curve, even in patients with no disease affecting the spleen. Spleen weight/volume among populations is non-Gaussian and skewed towards higher values; the usual definition of ‘normal’ as within two standard deviations of the mean does not apply.
Most radiologists base their assessment of splenomegaly on an ‘eyeball’ test or a maximal linear measurement. A study of patients in Crete proposed 315 cc as an upper limit of normal for spleen volume.3 An analysis on Brazilian patients predicted that any spleen with cephalocaudal dimension on CT greater than 10 cm would exceed this 315 cc limit.4 Regional differences among patient populations may render generalization of reported standards a difficult proposition; a 10 cm upper limit of normal would likely result in overly sensitive interpretation in American populations. A recent sonography study based on German stem cell donors has provided more realistic estimates of the 95th percentile for cephalocaudal spleen length: 13.6 cm for a 6-foot tall man, 12.4 cm for a 5-foot-8-inch tall woman. 5
Mononucleosis
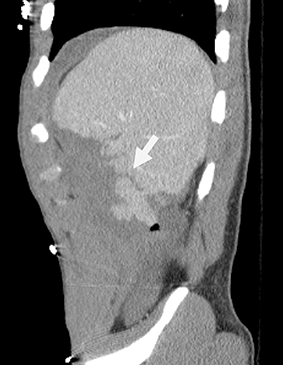
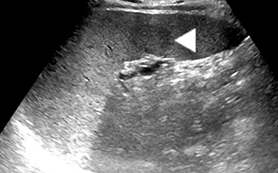
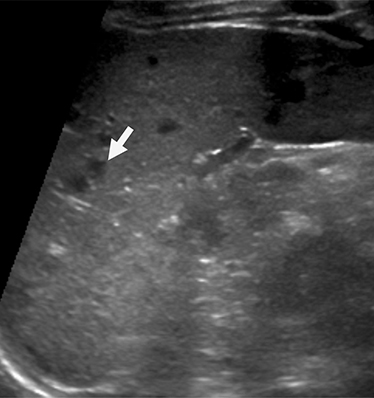
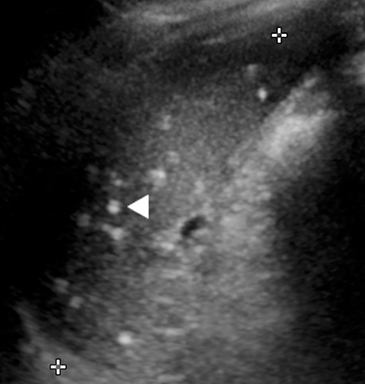
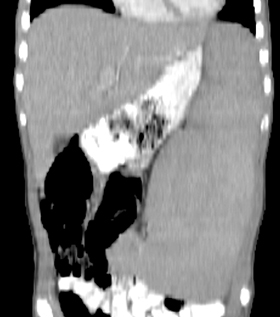
Mononucleosis is among the most common causes of splenomegaly among adolescents and young adults, as splenomegaly occurs in the majority of cases.6 The diagnosis is established clinically and confirmed with a serologic test. Few young patients with mononucleosis require medical imaging, although patients may present with symptoms and complications of splenic rupture after suffering trauma to their fragile, enlarged spleen (Figure 1).7
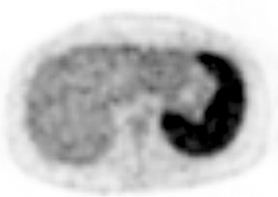
Mononucleosis in contact sport athletes requires special consideration; some sports physicians advise athletes have sonography before return to participation.8 When imaging is performed, the spleen is usually enlarged without focal lesions, although infarcts or focal hypertrophy of white pulp follicles may lead to focal lesions that resolve (Figure 1). Mononucleosis will transiently increase metabolic activity in the spleen, which may occasionally be encountered on18 F-2-fluoro-2-deoxy-glucose positron emission tomography (FDG-PET) scans performed in young patients for other reasons (Figure 1).
Pyogenic abscess
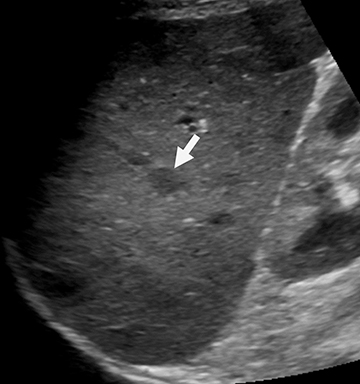
Pyogenic organisms spread to the spleen either hematogenously or by direct extension from adjacent infection.9 Splenic infarcts may be infected either by the emboli that caused them (eg, from endocarditis) or by secondary blood-born infection of a pre-existent defect (Figure 2). Large liquefied splenic abscesses can often be drained percutaneously, either with curative intent or as a temporizing measure prior to surgical treatment.
Cat scratch disease
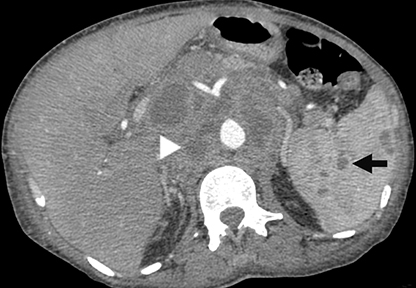
Bartonella species cause the bacterial granulomatous infection known as cat scratch disease. The typical patient is an immunocompetent adolescent with a cat. Cat scratch disease produces focal hepatosplenic lesions less than 2 cm in size (Figure 3). Often, these lesions have necrosis visible on MR as centrally prolonged T2. There may be peripheral ring-like enhancement. Most cases resolve spontaneously, usually before the disease is confirmed serologically. In rare cases cat scratch disease can present with larger, pyogenic abscesses. Initial lesions may evolve to echogenic, presumably calcified splenic lesions (Figure 3). However, the pattern of multiple small, calcified, granulomas is typically due to endemic fungal infections, such as histoplasmosis in the northeastern United States.
In immunocompromised patients, particularly those with AIDS, Bartonella produces a systemic infection with nodal, visceral and cutaneous involvement. In this setting the disease is known as bacillary angiomatosis (BA) or peliosis (BP). Even histologically, the lesions of BA resemble those of Kaposi’s sarcoma, which occurs in the same population.
Microabscesses
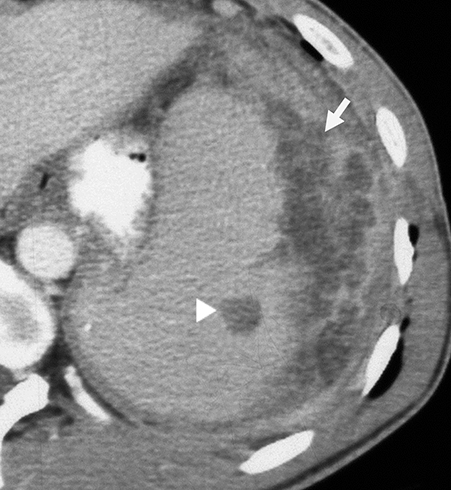
Tuberculosis constitutes a continuing worldwide threat. Disseminated tuberculosis can lead to visceral microabscesses.10 On sonography these can appear as tiny hypoechoic defects (‘salt and pepper’ pattern) of the spleen and/or liver (Figure 4). Other lesions may be large enough for central necrosis to be visible. On CT the lesions tend to be uniform in size, often involving both the liver and the spleen. Often, too, there is concomitant lymphadenopathy (Figure 4). Tuberculosis can disseminate in both immunocompetent and compromised patients.
Visceral fungal microabscesses arise almost exclusively in immunocompromised patients, including in patients with iatrogenic immune suppression, such as for treatment of acute myelogenous leukemia or after organ transplantation. Most fungal lesions are small and uniform. On MR there is usually enough central necrosis for T2 hyperintensity to be visible. However, there is little if any peripheral enhancement. As inflammatory response of the host is necessary in order for visceral fungal microabscesses to be visible on imaging, imaging may thus underestimate the extent of disease while the patient is most severely ill. As patients’ immune response returns, findings of immune reconstitution inflammatory syndrome can be seen, with enlarged or more numerous lesions even as the patient improves clinically.11
Patients who have excess splenic iron deposition, such as from hemolyzed red cells via repeated transfusion, will have abnormally short splenic parenchymal T2 and T2*. As a result, focal lesions such as microabscesses are readily detected in these patients against their dark spleen background. This makes MR particularly useful to detect microabscesses.
Approach to the immunocompromised patient with focal splenic lesions
An organized approach to the evaluation of focal splenic lesions in the immunocompromised patient has been previously described by Radin.12 Certain etiologies predispose to the presentation of lesions less than one centimeter and others that are greater than two centimeters (Table 1). Tuberculosis and lymphoma are considerations in both groups. Nearly half of all patients with splenic lesions larger than 2 cm were attributed to infection by Pneumocystis jirovecii (Figure 5). Pneumocystis should be considered in the differential diagnosis of multiple large splenic lesions with a sizable zone of central necrosis or hemorrhage out of proportion to the ring of solid peripheral tissue.
Visceral infections related to bioterrorism
Among the categorical lists of potential bioterrorism pathogens monitored by the Centers for Disease Control (CDC), Category B agents have less potential for human morbidity and are more difficult to disseminate than those in Category A. Category B includes three bacterial infections that can cause visceral abscesses, including of the spleen.13
Melioidosis is caused by Burkholderia pseudomallei.14 Endemic to the soil in Southeast Asia and Australia, these bacteria enter the human body through cuts in the feet, initially causing pneumonia, and ultimately resulting in irregularly-shaped low attenuation visceral lesions (Figure 6). Melioidosis spreads along catheter tracts; consequences of iatrogenic dissemination must be considered prior to any intervention.
Glanders, caused by the closely related Burkholderia mallei, is a disease of horses and horse handlers that has the dubious distinction of having actually been used as a weapon of biological warfare, against horse transport during the First World War. A case of glanders was reported in a research microbiologist in 2001; otherwise, there has not been a natural occurrence of glanders in the United States since 1949.15
Brucellosis, caused by bacteria of the genus Brucella, is primarily a disease of barnyard animals, although human cases also occur naturally. During acute disease the organism spreads throughout the reticuloendothelial system (RES). Radiologists in endemic areas recognize prior infections by large coarse splenic calcifications readily visible on plain radiographs.
Hydatid disease
Echinococcus granulosa rarely involves the spleen without also occurring in the liver. The typical splenic lesion, like hydatid cysts elsewhere, is a large, sharply-defined, multilocular cyst with multiple peripheral daughter lesions surrounding a central matrix.16 Smaller lesions may be simple, and some lesions particularly after treatment can be nearly solid. Cysts with dense peripheral calcification are considered dead, but adjacent tissue should be carefully inspected for local extension of an active daughter lesion. Hydatid cysts must be differentiated from other splenic cysts, as simple cysts can be multilocular and post-traumatic cysts often calcify.
Other parasitic infections
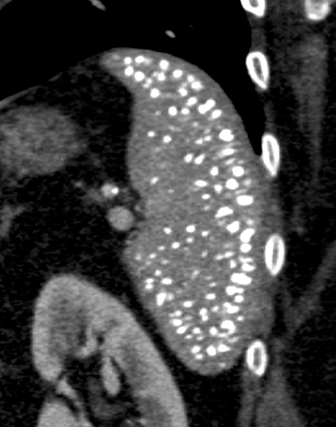
Many acute infections produce splenomegaly without focal lesions. Besides mononucleosis, these include typhoid fever, cytomegalovirus, plague, sepsis, malaria and kala azar.Malaria, caused by protozoan parasites of the genus Plasmodium, totals more than 200 million annual cases worldwide. Imaging typically shows mild to moderate splenomegaly. The diagnosis is made by examination of a peripheral buffy coat blood smear. Complications involving the spleen include infarcts and a peculiar ‘onion skin’ pattern of repeated peripheral hemorrhage that occurs in endemic areas. Deposition of hemozoin malaria pigment in the spleen can produce a speckled appearance of the spleen on ultrasound (Figure 7), mirroring the speckled histologic pattern known by the German term ‘fleckmilz,’ or ‘spleen spots.’17
Visceral leishmaniasis, caused by protozoan parasites of the genus Leishmania, is spread by sandflies. Half of the 12 million cases worldwide, predominantly cutaneous, occur in the Indian subcontinent. In the Americas, cases are reported as far north as Texas. The disease is sometimes called kala azar, meaning “black skin” in an Indian dialect, which refers to the grey pallor of its victims. The spleen and other tissues in the RES are filled with macrophages packed with parasites; marrow infiltration leads to profound anemia. When imaging is performed, the spleen is massively enlarged, sometimes with tram-track calcifications along the intra-splenic vasculature.
Autoimmune diseases
Various autoimmune conditions can produce splenic findings. In patients with lupus erythematosis, there may be diffuse punctate calcifications (Figure 7), although the pattern of numerous tiny calcifications also can occur after pneumonia. Affected spleens may resemble those of sickle cell patients undergoing autosplenectomy, but the spleen is not typically small. Rapid enlargement of the spleen in a lupus patient should raise concern for the possibility of lymphoma.
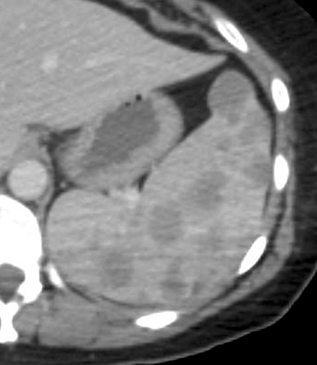
Another autoimmune condition involving the spleen is Felty’s syndrome occurring in the setting of rheumatoid arthritis. In Felty’s syndrome the spleen rapidly enlarges (Figure 8), sequestering white blood cells, leaving the patient with profound immune compromise from neutropenia. Resultant spleen size does not correlate with the degree of hematologic abnormality; without splenectomy, death results from overwhelming bacterial infection.18 Therefore, it is important to recognize rapid splenic enlargement in the setting of rheumatoid arthritis.
Finally, patients with granulomatosis with polyangiitis (previously Wegener’s granulomatosis) can have a peculiar central splenic infarct.19 The spleen has an irregular shape, retracted around a central scar-like area of low attenuation.
Sarcoidosis
Histologically, sarcoidosis affects the spleen in approximately 24-59% of patients, with non-caseating granulomas.9 In the majority of patients the granulomas are too small to be visible on imaging. In 15% of cases, there are focal lesions ranging in size from punctate up to 3 cm, representing coalescent granulomas (Figure 9). Lesions are hypoattenuating on CT. On MR they are low signal intensity relative to surrounding spleen on both T1 and T2 weighted images, enhancing slowly and weakly.0 In severe cases sarcoid lesions are nearly coalescent.
In some patients abdominal imaging may be the first indication of sarcoidosis. In the spleen, sarcoidosis typically produces a pattern of splenic involvement with numerous small uniform focal lesions. Sarcoidosis must be distinguished from lymphoma, which may have a similar appearance. As both sarcoidosis and lymphoma can lead to focal or diffuse splenic hypermetabolism, FDG-PET scans do not help to distinguish sarcoid from lymphoma (Figure 9); however, diffuse or multifocal lymphomatous splenic involvement rarely occurs without lymphoma elsewhere.
Rarely, a sarcoid-like reaction has been reported to arise in patients with malignancy, who have been treated with chemotherapy (Figure 10).21 Often these sarcoid-like findings have resolved after completion or suspension of therapy, with or without additional treatment with steroids. Sarcoid-like reaction has also been reported after monoclonal antibody therapy for inflammatory conditions like rheumatoid arthritis.22
Inflammatory pseudotumor and SANT
Inflammatory pseudotumor occurs in the spleen as well as other organs. Histologically, there will be variable amounts of hemorrhage, inflammatory cells, and fibrosis. Therefore, the imaging appearance can be similarly variable. The lesions available for review have been well-defined, usually with gadolinium enhancement, and heterogeneous signal intensity on unenhanced images.23
Sclerosing adenomatoid nodular transformation, SANT, is a recently recognized histologic entity.24 Histologically, SANT consists of a well-defined but non-encapsulated mass, with a nodular growth pattern of vascular spaces surrounded by fibrosis with inflammatory cells. Its pathogenesis is unknown. On imaging, SANT is a solitary, well-defined splenic mass with T2 longer than adjacent splenic parenchyma. Often there is a spoke-wheel pattern of enhancement. Some cases have restricted diffusion, and some have hypermetabolism on FDG-PET. SANT is not considered a neoplasm, but is usually resected as its confident diagnosis is rarely made by imaging.
Conclusion
If there is an overriding consideration in confronting imaged splenic abnormalities, it is to consider the entirety of a patient’s clinical condition. Beyond demographic information, travel and environmental exposure history may be helpful for proposing differential infectious possibilities. Laboratory studies and treatment history may be helpful in proposing potential inflammatory etiologies of splenic findings.
References
- Heymsfield SB, Fulenwider T, Nordlinger B, et al. Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Intern Med. 1979;90(2):185-187.
- Geraghty EM, Boone JM, McGahan JP, et al. Normal organ volume assessment from abdominal CT. Abd Imag. 2004;29(4):482-490.
- Prassopoulos P, Daskalogiannaki M, Raissaki M, et al. Determination of normal splenic volume on computed tomography in relation to age, gender and body habitus. Eur Radiol. 1997;7(2):246-248.
- Bezerra AS, D’Ippolito G, Faintuch S, et al. Determination of splenomegaly by CT: is there a place for a single measurement? AJR Am J Roentgenol. 2005;184(5):1510-1513.
- Chow KU, Luxembourg B, Seifried E, et al. Spleen size is significantly influenced by body height and sex: established of normal values for spleen size at US with a cohort of 1200 healthy individuals. Radiology. 2016;279(1):306-313.
- Dommerby H, Stangerup S-E, Stangerup M, et al. Hepatosplenomegaly in infectious mononucleosis, assessed by ultrasonic scanning. J Laryng Otol. 1986;100(5):573-580.
- Cruz-Romero C, Agarwal S, Abujudeh HH, et al. Spleen volume on CT and the effect of abdominal trauma. Emerg Radiol. 2016. Published online May 11, 2016.
- Becker JA, Smith JA. Return to play after infectious mononucleosis. Sports Health. 2014;6(3):232-238.
- Robertson F, Leander P, Ekberg O. Radiology of the spleen. Eur Radiol. 2001;11(1):80-95.
- Burrill J, Williams CJ, Bain G, et al. Tuberculosis: a radiologic review. RadioGraphics. 2007;27(5):1255-1273.
- Rajeswaran G, Becker JL, Michailidis C, et al. The radiology of IRIS (immune reconstitution inflammatory syndrome) in patients with mycobacterial tuberculosis and HIV co-infection: appearances in 11 patients. Clin Radiol. 2006;61(10):833-843.
- Radin R. HIV infection: analysis in 259 patients with abnormal abdominal CT findings. Radiology. 1995;197:712-722.
- National Institute of Allergy and Infectious Diseases. “NIAID Emerging Infectious Diseases/Pathogens.” www.niaid.nih.gov/topics/biodefenserelated/biodefense/pages/cata.aspx Last updated: January 26, 2016. Accessed: June 14, 2016.
- Benoit TJ, Blaney DD, Doker TJ, et al. A review of melioidosis cases in the Americas. Am J Trop Med Hyg. 2015;93(6):1134-1139.
- Srinivasan A, Kraus CN, DeShazer D, et al. Glanders in a military research microbiologist. N Engl J Med. 2001;345(4):256-258.
- Pedrosa I, Saiz A, Arrazola J, et al. Hydatid disease: radiologic and pathologic features and complications. RadioGraphics. 2000;20(3):795-817.
- Schmeisser HC, Harris Jr LC. Multiple necroses of the spleen (Fleckmilz). Am J Pathol. 1938;14(6):821-830.
- Fishman D, Isenberg DA. Splenic involvement in rheumatic diseases. Semin Arthritis Rheum. 1997;27(3):141-155.
- Fonner CT, Nemcek AA, Bochman C. CT appearance of splenic infarction in Wegener’s granulomatosis. AJR Am J Roentgenol. 1995;164(2):353-354.
- Warshauwer DM, Semelka RC, Ascher SM. Nodular sarcoidosis of the liver and spleen: appearance on MR images. J Magn Reson Imaging. 1994;4:553-557.
- Chowdury FU, Sheerin F, Bradley KM, et al. Sarcoid-like reaction to malignancy on whole-body integrated18F-FDG PET/CT: prevalence and disease pattern. Clin Radiol. 2009;64(7):675-681.
- Ramos-Casal M, Perez-Alvarez R, Perez-de-lis M, et al. Pulmonary disorders induced by monoclonal antibodies in patients with rheumatologic autoimmune diseases. Am J Med. 2011;124(5):386-394.
- Hedgire SS, Sainani NI, McDermott S, et al. Mono-belly and beyond: spectrum of imaging manifestations of EBV infection in the abdomen. Clin Imag. 2013;37(4):711-717.
- Raman SP, Singhi A, Horton KM, et al. Sclerosing angiomatoid nodular transformation of the spleen (SANT): multimodality imaging appearance of five cases with radiology-pathology correlation. Abd Imag. 2013;38(4):827-834.
Citation
LK L, PF H.Beyond splenomegaly: An image-based review of infectious and inflammatory diseases of the spleen. Appl Radiol. 2017; (7):24-29.
July 1, 2017