An image-based review of the ACR Appropriateness Criteria for Low Back Pain
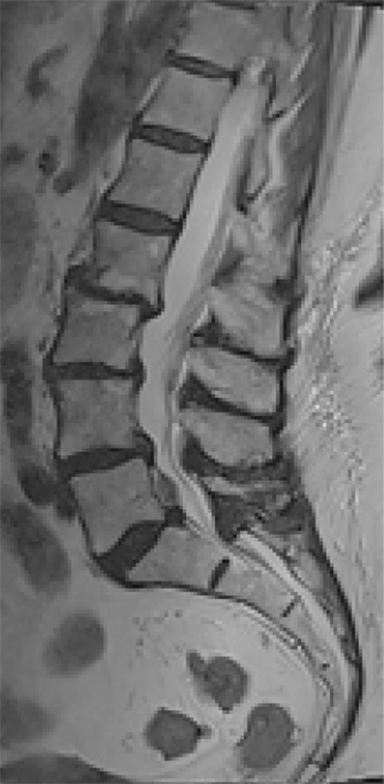
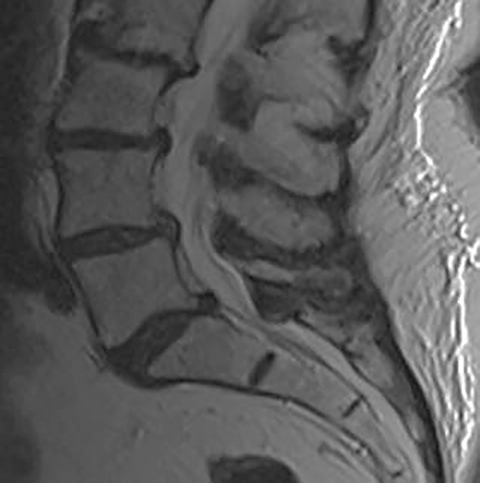
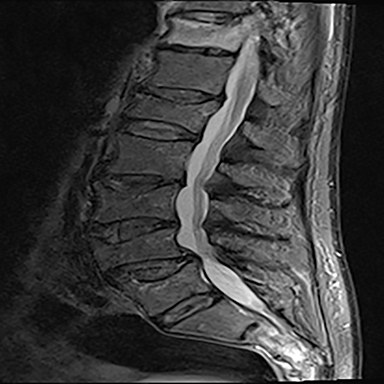
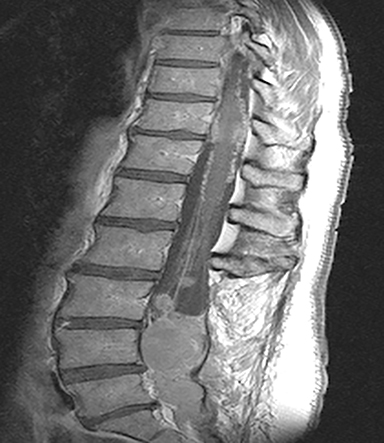
Low back pain (LBP) is one of the most common medical complaints in the United States, with estimates of between 70% and 80% of Americans reporting at least one episode of back pain during their lifetime, and 2% to 5% of patients seeking annual medical attention for the condition.1 LBP is the fifth-most common reason for physician visits in the U.S.2
National estimates for direct expenditures for LBP, based on the Medicare Expenditures Panel Survey, range from $46 billion to $102 billion USD from 1997 to 2005. These figures represent approximately 1/10th of overall U.S. medical expenditures, with a generally increasing trend over that period.3 These estimates do not take into account indirect costs in the form of lost productivity at work, time taken off from work, disability, and decreased quality of life.4
Medical imaging, in particular cross-sectional modalities such as MRI and CT, offers unparalleled ability to identify, characterize, and distinguish the myriad pathologies that can contribute to LBP. Inherent limitations, however, do exist. In the general population, the prevalence of lumbar degenerative changes observable on imaging is high, and there are only limited direct correlates between such findings and symptoms. The value of diagnostic imaging is in the identification of pathology to guide management and impact outcome. Because of the mostly self-limited nature of uncomplicated LBP, early imaging has not been found to reliably impact outcome.4
Meanwhile, the frequency of diagnostic imaging studies for LBP has increased in recent decades, with a simultaneous shift towards higher-cost cross-sectional imaging.4 In this era of healthcare reform with stress on patient outcomes, value-added benefit, cost-savings, and the move toward bundled reimbursements, it is critical for radiologists to help guide the appropriate application of diagnostic imaging. Routine imaging for LBP without any concomitant risk factors has specifically been singled out as the most commonly ordered diagnostic test or treatment lacking in proven efficacy and a “most egregious [cause] of waste” by the National Physicians Alliance, a group composed of internal medicine and family medicine practitioners.5
The American College of Radiology (ACR) Appropriateness Criteria for Low Back Pain serves as a reference to guide both referring physicians and radiologists toward best practices for ordering studies. These guidelines maximize the benefit of powerful imaging studies by applying them to the correct clinical situation. The ACR criteria are composed of evidence-based ratings (scored 1 to 9) of different imaging modalities in terms of utility, sorted by indication. Overall, the criteria are derived from the work of more than 300 volunteer physicians with expertise in the related imaging modalities and/or clinical settings, including more than 80 from non-radiology experts. The criteria follow defined attributes for medical practice guidelines as set by the Institute of Medicine and the Agency for Healthcare Research and Quality.6
The ACR Practice Parameters are a complementary set of documents that describe common indications, findings, and technical parameters on a modality- and indication-specific basis. For LBP, separate Practice Parameter documents are available for radiography, CT, and MRI.7 As with the ACR Appropriateness Criteria and Practice Parameter documents on other topics, the criteria presented for LBP are guidelines, explicitly advising that ultimate referrer decisions on imaging should be made on the basis of the complexity, severity, and other circumstances specific to an individual case. The Appropriateness Criteria were last reviewed and updated in 2015.7, 8
The following is an image-based review of findings, indications, and specific higher-risk scenarios (so-called “red flags”) as discussed in the Appropriateness Criteria for LBP.
ACR Appropriateness Criteria for Low Back Pain
The central theme of the current criteria for imaging LBP is the recognition that acute, uncomplicated LBP is typically a self-limited phenomenon, without supporting evidence that early imaging leads to better outcomes.1, 4 An often-cited meta-analysis of six randomized, controlled trials found no significant difference between immediate imaging and routine supportive care without imaging, for all three major imaging modalities (radiography, CT, and MR). Outcome measures included pain and function in the short and long term, quality of life, mental health, and overall improvement.9
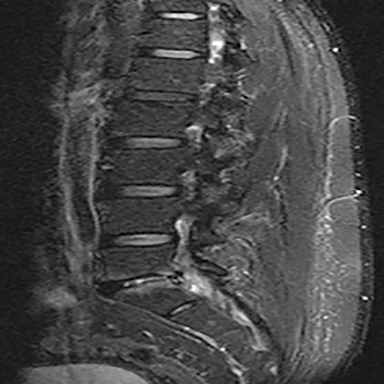
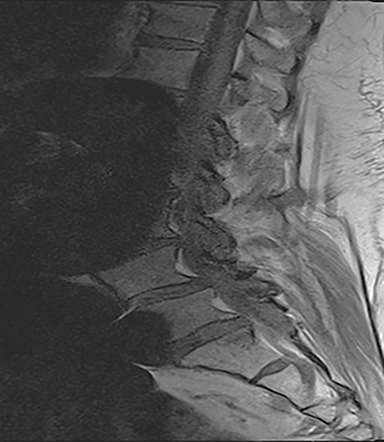
The reported natural history of uncomplicated back pain is that 50% of patients with acute LBP recover after 2 weeks and 90% after 3 months.1 Uncomplicated acute LBP may be treated conservatively with pain control, reassurance, and physical therapy without initial imaging, according to standard clinical guidelines.8 As a result, for uncomplicated acute LBP lasting less than 6 weeks, the Appropriateness Criteria scores for all imaging modalities are either 1 or 2, defined as “usually not appropriate”8 (Figure 1).
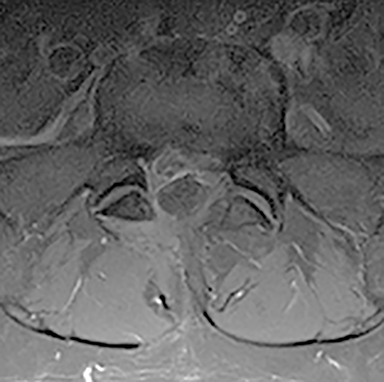
Early imaging is appropriate for specific indications. In addition to pain lasting longer than 6 weeks of conservative management, other “red flags” include: trauma, unexplained weight loss, minor fall or heavy lift in a potentially osteoporotic or elderly individual, fever/infection, immunosuppression, cancer, IV drug use, prolonged corticosteroid use, focal neurologic deficit with progressive or disabling symptoms (including those of cauda equina syndrome), and/or prior surgery.8 For these categories, MRI is generally rated as 7-8, defined as “usually appropriate.”
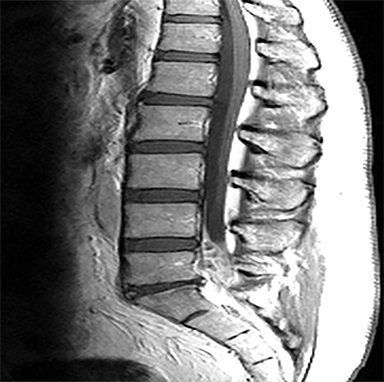
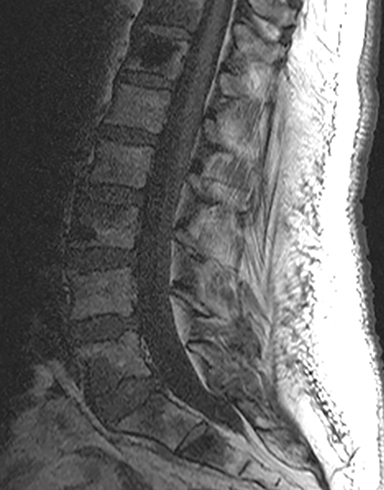
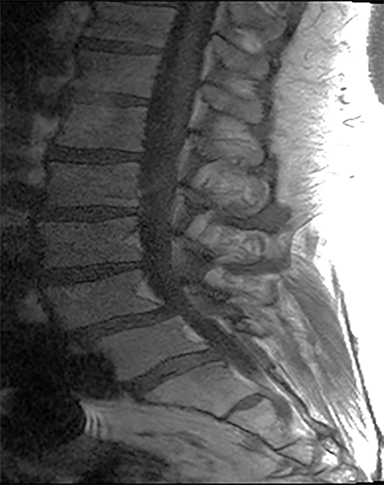
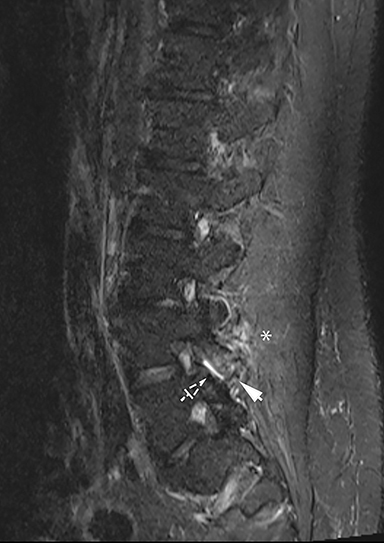
The red flag indications can be subdivided by whether the indication calls for contrast-enhanced imaging. Indications including trauma, osteoporosis/age, focal/progressive deficit, and chronic steroid use call for initial evaluation with X-ray of the lumbar spine, CT for detailed osseous evaluation of possible vertebral body fracture, and MRI for ligamenous injury, worsening neurologic deficit, and for marrow edema, rating all three modalities as a 7 or “usually appropriate,” (Figure 2). Indications consisting of suspicion of cancer, infection, and/or immunosuppression correspondingly raise appropriateness scores of contrast studies (Figures 3 and 4).
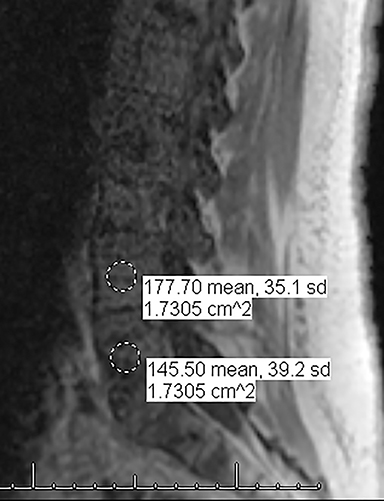
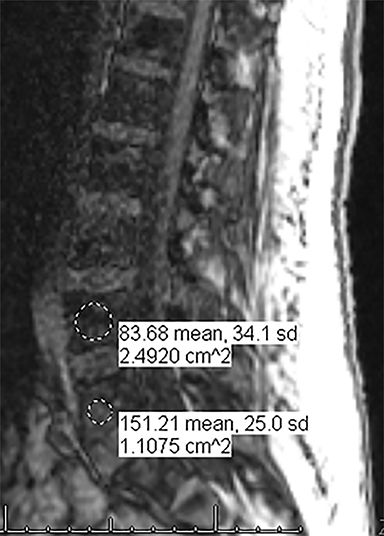
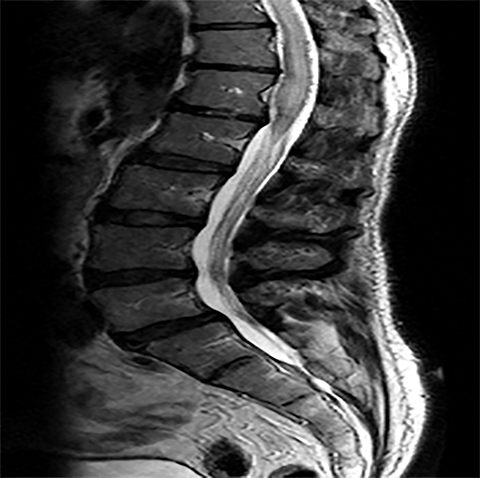
For evaluating marrow replacement processes such as metastatic disease, in- and out-of phase MR imaging can be employed. The normal marrow undergoes fatty conversion and results in a diffuse dispersion of fat. This causes a combination of fat and water within an imaging voxel. Fat and water protons precess at slightly differing frequencies, and the averaged signal intensity within the voxel will vary according to TE, depending on whether the protons are in or out of phase. An invariant voxel implies contents other than typical marrow (Figure 5).
The Appropriateness Criteria for any LBP indication outside of the six-week initial presentation period rates CT as 5-6 (may be appropriate), specifying that it is useful if MRI is unavailable, contraindicated, or indeterminate. CT can also be useful for problem solving and gives complementary information particularly in preoperative patients.8 In particular, CT adds information on hypertrophic bone, trabecular pattern, pathologic fracture, and sclerotic metastases.
The Appropriateness Criteria document provides a separate table for patients with LBP and/or radiculopathy who are surgical candidates. Under this rating system, discography is marginally higher but rated as “3” (usually not appropriate). Potential benefits of injection discography include localization of pathology via reproduction of symptoms, characterization of disc defects, and characterization of features associated with progressive versus injury-related pain.10 Discography may offer an alternative for patients with MRI contraindications. Other studies have demonstrated less consistent findings,11 and the frequency of performing this study varies with referring physician practice patterns.
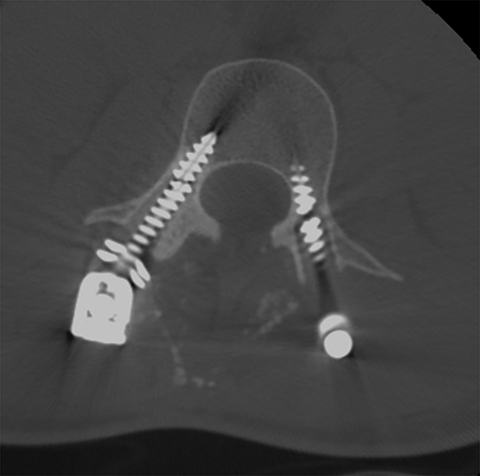
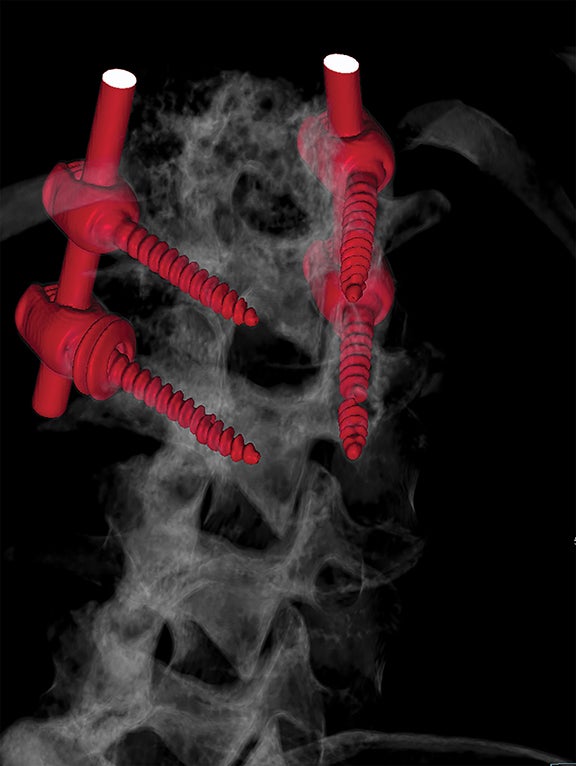
For patients with previous lumbar surgery, radiography can help to evaluate hardware integrity. CT offers a superior evaluation of osseous fusion (which occurs in 95% of cases by 6 months), and allows evaluation of surgical hardware. MRI with and without contrast remains the highest-rated modality with a score of “8.” The usefulness of contrast MRI in this setting includes the ability to evaluate infection, recurrent disc protrusions, and postsurgical scar (Figures 6 and 7).
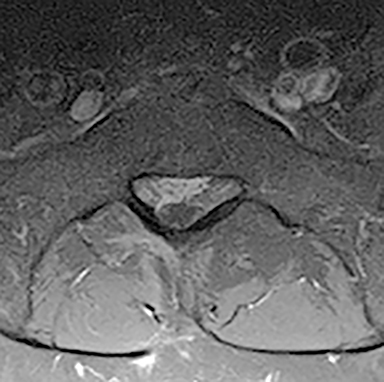
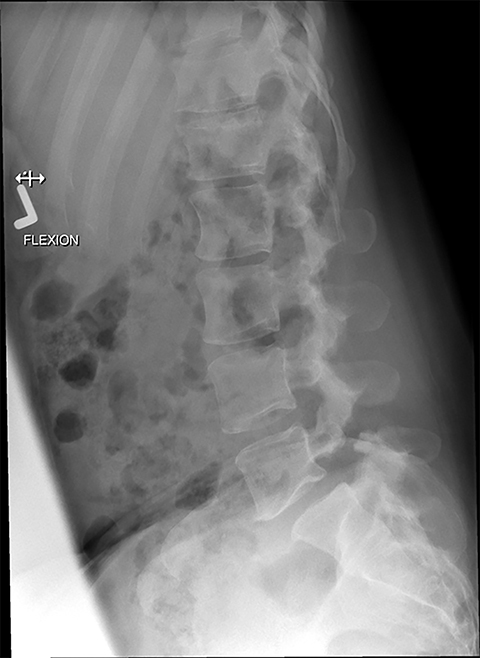
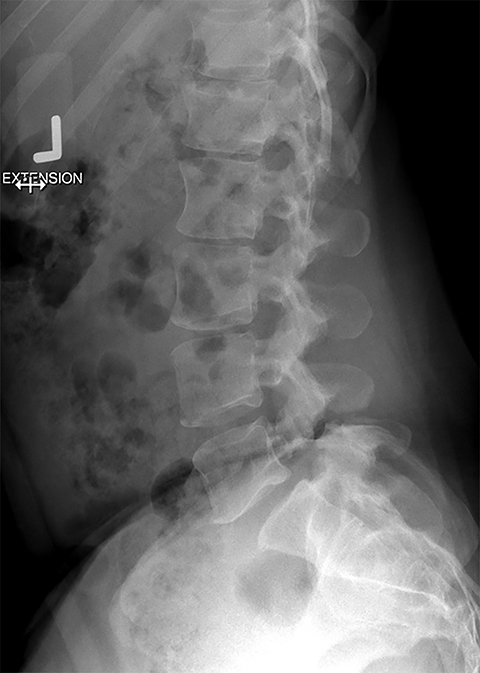
Presurgical patients generally do not benefit from conventional radiography, as reflected in Appropriateness Criteria ratings. Exceptions include patients undergoing evaluation of dynamic instability via flexion and extension views (Figure 8). For cauda equina and multifocal/progressive deficit patients, MR offers a superior evaluation of the cauda equina in comparison to conventional CT (Figures 3 and 9). These patients, as well as presurgical and postsurgical patients, may benefit from myelography in certain situations.8
Metal artifact in the postsurgical patient can present a dilemma in imaging selection between MRI and other modalities such as post-myelography CT. Newer MRI techniques such as Slice Encoding Metal Artifact Correction (SEMAC) and View Angle Tilting (VAT), as well as parameter optimization (TSE, short echo train length, not using parallel imaging, increased bandwidth and number of excitations, thinner slices, no fat saturation, etc.) can decrease susceptibility artifact, which in turn may obviate the invasiveness and radiation dose associated with myelography (Figure 10). Many additional techniques are described elsewhere in the literature, including parallel imaging, CSF flow imaging, T1-FLAIR, perfusion, dynamic imaging and upright MRI.7
Discussion
The ACR Appropriateness Criteria for LBP affirms that imaging is generally not indicated for uncomplicated LBP of less than 6 weeks, in particular for nonsurgical patients. The goal for imaging remains to evaluate patients with red flags, such as prolonged symptoms, radiculopathy, history of trauma, osteoporosis, cancer, infection, immunosuppression, or symptoms related to the cauda equina. For these patients, MRI is the preferred modality.
CT and other modalities such as myelography can offer complementary information or act as an alternative if MRI is contraindicated. Plain radiography may benefit the patient in specific situations, such as a limited evaluation for alignment or dynamic instability. Optimal postsurgical imaging varies. For instance, MRI with contrast is superior for evaluating residual disc versus granulation scar, while CT is superior for evaluating postsurgical osseous fusion. Contrast enhancement will typically aid the patient with a history or suspicion of neoplasm, infection/inflammation, or immunosuppression.
Several studies have evaluated the apparent efficacy of LBP clinical guidelines and their adoption by clinicians. Although practice guidelines have been established since 2007 obviating the need for early MRI in cases of uncomplicated LBP, observational studies from 2010-2014 show that up to 20% of such patients still receive early MRI. Patients receiving unnecessary early MRI continue to be associated with higher overall costs (in one study, $22,151 vs $6,640) and rates of surgery (19.9% vs 2.5%).12
Non-adherence to guidelines may be due in part to communication and education. A 2010 Cochrane study reviewed the interventions that have been employed to increase physician guideline adherence. They found that most involved distribution of educational material and had no demonstrable impact on overutilization.13 A more effective method of increasing compliance with following LBP imaging guidelines may be through the implementation of IT-enabled clinical decision support systems. In one study, implementation of such a system resulted in a 30.2% reduction in primary care lumbar spine MRI orders, and guideline adherence similarly increased from 78% to 96%.14 Other factors that may explain early MRI ordering for LBP include patient expectations, patient satisfaction, and medico-legal concerns.2
Current directions in imaging research address evidence-based imaging contributions towards outcomes. One shortcoming of lumbar spine imaging is that imaging frequently yields findings of unknown clinical significance.4 Many groups have attempted to address this question by investigating the symptomatic value of individual imaging findings. Recently, Goode et al published a meta-analysis of 28 studies focusing on radiographs, encompassing 26,107 patients. They identified several findings significantly associated with symptoms, including disc space narrowing, spondylolisthesis, spondylosis, and osteophytes, while finding no significant association for endplate sclerosis and facet osteoarthrosis.15 Studies such as these can help inform our reporting.
On the other hand, a smaller study (on a Framingham Offspring Cohort subpopulation undergoing CT) found a positive association between severe facet osteoarthritis and clinical symptoms,16 suggesting that optimal evaluation of specific findings is dependent on imaging technique. This study corroborates prior reports17,18 showing that severity, rather than mere presence, of findings provides better correlation to symptoms. In a separate article, the same group demonstrated increased odds ratios of LBP for individual findings of facet bone marrow lesions, facet effusions, and high periarticular signal intensity.19
The Framingham Offspring Cohort CT study additionally detected an association between symptomatic disc space narrowing and age less than 60 yr. Because their study population included both middle-aged and elderly patients, this provides evidence that symptomatic disc space narrowing and facet OA may have different age distributions, and supports the concept that LBP-related findings transition from the disc to the facet joints with age (Figure 11).16, 20
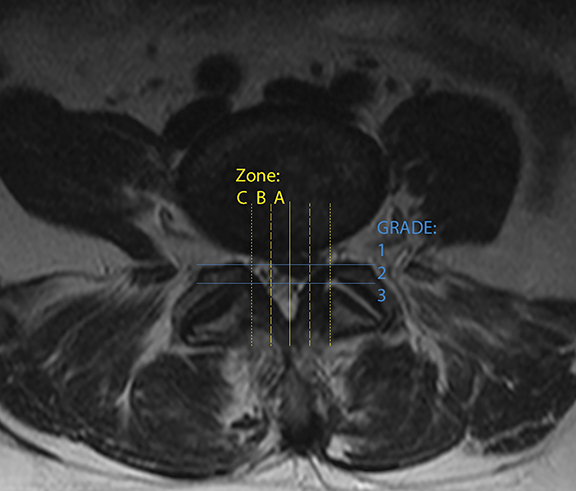
Lastly, interobserver variability is an often-cited impediment to the effective imaging of LBP.21 Numerous grading scales exist for imaging findings, as well as for the evaluation of pain and disability. A recent review of different imaging grading scales reported that interobserver kappa scores ranged all the way from 0.28 to 0.81. However, efforts to achieve greater uniformity have been successful with the development of reproducible grading systems such as The Combined Task Force nomenclature for lumbar disc disease (updated in 2014)22 and the van Rijn criteria for lumbar nerve root compression. Both demonstrated higher interobserver kappa scores compared to alternatives and were found to be among the most commonly used. A third, newer nomenclature system from Michigan State University,23 based on discriminating and localizing larger herniations for surgical optimization, showed 98% interobserver concordance (Figure 12).21
Conclusion
In this era of high healthcare costs, the optimal use of appropriate diagnostic imaging is critical. As radiologists, we have a responsibility to champion appropriate imaging, as this adds critical value to our work and profession. The ACR Appropriateness Criteria as well as other similar guidelines help identify higher-risk individuals, and aid in providing recommendations for the best imaging modality for LBP in a variety of clinical scenarios. Further goals for the radiology community should include improving adherence to standard reporting nomenclature and continued high-quality research to correlate individual imaging findings to symptoms and outcomes.
References
- Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25(2):353-371.
- Srinivas SV, Deyo RA, Berger ZD. Application of “less is more” to low back pain. Arch Intern Med. 2012;172(13):1016-1020.
- Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and expenditures and health status among adults with back and neck problems. JAMA. 2008;299(6):656-664.
- Chou R, Deyo RA, Jarvik JG. Appropriate use of lumbar imaging for evaluation of low back pain. Radiol Clin N Am. 2012;50(4):569-585.
- Good Stewardship Working Group. The “top 5” lists in primary care: meeting the responsibility of professionalism. Arch Intern Med. 2011;171(15):1385-1390.
- American College of Radiology. ACR Appropriateness Criteria Overview. Feb 2015.
- American College of Radiology. ACR-ASNR-SBCT-MR Practice Parameter for the Performance of Magnetic Resonance Imaging (MRI) of the Adult Spine. Res19-2012, Amended 2014 (Res.39)
- American College of Radiology. ACR Appropriateness Criteria for Low Back Pain. Revised 2015.
- Chou R, Fu R, Carrino JA, et al. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463-472.
- Bartynski WS, Dejohn LM, Rothfus WE, et al. ‘Progressive-onset’ versus injury-associated discogenic low back pain: features of disc internal derangement in patients studied with provocation lumbar discography. Interv Neuroradiol. 2013;19(1):110-120.
- Siepe CJ, Heider F, Haas E. Influence of lumbar intervertebral disc degeneration on the outcome of total lumbar disc replacement: a prospective clinical, histological, X-ray and MRI investigation. Eur Spine J. 2012;21(11):2287-2299.
- Graves JM, Fulton-Kehoe D, Jarvik JG, et al. Health care utilization and costs associated with adherence to clinical practice guidelines for early magnetic resonance imaging among workers with acute occupational low back pain. Health Serv Res. 2014;49(2):645–665.
- French SD, Green S, Buchbinder R, et al. Interventions for improving the appropriate use of imaging in people with musculoskeletal conditions. Cochrane Database Syst Rev. 2010; 1.
- Ip IK, Gershanik EF, Schneider LI, et al. Impact of IT-enabled intervention on MRI use for back pain. Am J Med. 2014;127(6):512-518.
- Raastad J, Reiman M, Coeytaux R, et al. The association between lumbar spine radiographic features and low back pain: a systematic review and meta-analysis. Semin Arthritis Rheum. 2015; 44(5):571-585.
- Suri P, Hunter DJ, Rainville J, et al. Presence and extent of severe facet joint osteoarthritis are associated with back pain in older adults. Osteoarthritis Cartilage. 2013;21(9):1199-1206.
- Cheung KM, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine. 2009;34(9):934–940.
- Hancock MJ, Maher CG, Laslett M, et al. Discussion paper: what happened to the ‘bio’ in the bio-psycho-social model of low back pain? Eur Spine J. 2011;20(12):2105-2110.
- Suri P, Dharamsi AS, Gaviola G, et al. Are facet joint bone marrow lesions and other facet joint features associated with low back pain? A pilot study. PM R. 2013;5(3):194-200.
- Gellhorn AC, Katz JN, Suri P. Osteoarthritis of the spine: the facet joints. Nat Rev Rheumatol. 2013 Apr;9(4):216-224.
- Li Y, Fredrickson V, Resnick DK. How should we grade lumbar disc herniation and nerve root compression? A systematic review. Clin Orthop Relat Res. 2015; 473(6): 1896-1902.
- Fardon DF, Williams AL, Dohring EJ. Lumbar disc nomenclature: version 2.0: Recommendations of the combined task forces of the North American Spine Society, the American Society of Spine Radiology and the American Society of Neuroradiology. Spine J. 2014;14(11):2525-2545.
- Mysliwiec LW, Cholewicki J, Winkelpleck MD, et al. MSU classification for herniated lumbar discs on MRI: toward developing objective criteria for surgical selection. Eur Spine J. 2010;19(7):1087-1093.
