Gastrointestinal bleeding scintigraphy
Gastrointestinal bleeding (GIB) accounts for 20% of emergency room visits, 5% of ER admissions, and 2% of all hospital admissions.1 An important initial step in the evaluation of GIB involves determining whether the source of the bleed is proximal or distal to the ligament of Treitz, located at the junction between the fourth segment of duodenum and proximal jejunum.2 This defines the disease as upper or lower GIB, respectively. Upper GIBs, initially evaluated by nasogastric tube placement and esophagogastroduodenoscopy, are more common than lower GIBs, and have a mortality rate that is 2-3 times higher compared with LGI sources.1
Lower GIBs account for approximately 20% of all cases of acute GI hemorrhage, with an annual incidence of 20 per 100,000 people. Lower GIBs have a mortality rate of approximately 4%.3 While 85% of Lower GIBs stop spontaneously, it is important to identify and stratify the remaining 15% according to those patients who are at higher risk and who are likely to benefit most from timely intervention.4
Diverticular disease accounts for 17-40% of the cases of lower GIBs, and results from weakness at the site in the colon wall where the circular muscle layer is penetrated by the vas recta which drape over the dome of the diverticulum and become susceptible to trauma and disruption.5 A further 10-40% of lower GIBs are caused by angiodysplasia primarily of the cecum and right colon.3 The most common causes of lower GIBs are listed in Table 1.2
Radiopharmaceuticals
GIB scintigraphy can play an important and unique role in characterizing and risk stratifying patients presenting with lower GIB. The superior diagnostic efficacy of esophagogastroduodenoscopy makes the use of red blood cell (RBC) scintigraphy for the detection of upper GIB less advantageous, although proximal small bowel, duodenal and even distal gastric bleeds are often detected. Patient’s presenting with acute GIB usually undergo an initial clinical assessment and resuscitation, nasogastric lavage and esophagogastroduodenoscopy, which serve to stratify the bleeding into either suspected upper or lower GI sources.6 If a lower GIB is suspected or an upper GI source has been excluded GIB scintigraphy can be performed as the next step in the diagnostic evaluation. Both bleed location (or suspected location) and rate of bleeding help determine the therapeutic strategy utilized, with the three main interventional approaches being colonoscopy, angiography and surgery.4
Tc99m sulfur colloid (SC) was the first radiopharmaceutical used for evaluation of lower GIBs. It is cleared from the blood pool by 10-15 minutes after administration, with a half-life of 2 to 3 minutes.2 The ability of Tc99m SC to detect GIB detection is due to the high target to background ratio between the bleeding site and the surrounding soft tissues, caused by rapid clearance of the background by the RES cells in the liver, spleen and bone marrow. However, the rapid clearance of radiotracer from the blood pool means that the patient must be bleeding at the time of injection. Delayed imaging is not possible. Tc99m SC is now mostly used when there are time constraints in preparing Tc99m RBCs or a lack of availability. In a prospective study comparing Tc99m RBC to Tc99m SC scintigraphy in 100 patients imaged with both agents under identical clinical conditions, Tc99m RBCs was diagnostically superior in all cases with sensitivity of 95%, specificity of 93% and an overall accuracy of 94%. This is in contrast to a sensitivity of 12%, 100% specificity and 62% overall accuracy for sulfur colloid.6
There are various in vivo and in vitro methods available for labeling RBCs with Tc99m. An often used in vitro method involves utilization of a simple commercial kit and permits >97% labeling efficiency in less than 30 minutes preparation time. A minimum detectable bleeding rate of 0.04 mL/minute was reported for Tc99m RBCs. Detection of lower GIBs at low flow rate is influenced by the volume of extravasated RBCs at the bleeding site. A focal volume of 3 mL can be readily detected however the sensitivity of the procedure can be reduced by hyperactive peristalsis, which can cause the volume to be distributed over a significant length of bowel.3
The relatively stable persistence of Tc99m RBCs in the blood pool enables intermittent (and venous) bleeding to be detected, permitting repeat imaging without additional radiation exposure. RBC scintigraphy can be acquired for up to 24h, making it unique among diagnostic imaging methods in being able to provide monitoring of patients with intermittent bleeding.7
RBC scintigraphy bleeding scan will be negative unless the patient is actively bleeding. In patients with active, intermittent bleeding who are hemodynamically stable between episodes, RBC scintigraphy compliments clinical findings by possible localization of ongoing bleeding and risk assessment for future interventions, reducing the rate of negative angiograms from 22 to 53%.1
Tc99m RBCs accurately localize the site of bleeding in 88% to 97% of patients, with positive findings resulting in a 5-fold greater likelihood that the patient will require surgery.4 False positive scans, which can be avoided with proper methodology and interpretation, are generally due to incorrectly identified vascular structures or free 99m Tc pertechnetate.
RBC scintigraphic has been proven to be a useful early diagnostic tool in Lower GIB risk-stratification and in guiding decisions regarding surgical or angiographic intervention. This is critical in a disease process characterized by spontaneous cessation in 75-89% of cases and potential high mortality in those who continue to bleed. Equally important is that there is a significant risk associated with potential interventions such as angiography and surgery.8
A positive scan is predictive of increased hospital morbidity and mortality, helping to identify a relatively high risk population requiring more aggressive intervention such as transfusion or surgery.9 Conversely, a negative study has been shown to correlate with a good clinical outcome, stratifying those patients who can be managed conservatively. A negative study may play a role in identifying those patients who are not actively bleeding and, due to very low volume of blood loss or slow rate, are more likely to stop bleeding spontaneously. This prevents overaggressive surgical management and may lead to a reduction both in morbidity and mortality.10
Methodology and image interpretation
A typical RBC scintigraphy protocol for lower GIB involves imaging with a large-field-of-view gamma camera and high resolution, parallel-hole collimator.11 After intravenous injection of 20 mCi of Tc99m labeled RBCs, flow images are acquired for 60 seconds at a rate of 1-second per frame followed by dynamic imaging for up to 90 minutes at a rate of 1-minute per frame. A further 30 minutes of imaging at 1-minute per frame can be obtained up to 24 hours after radiotracer administration. Inefficient labeling can be evaluated by acquiring static images of the anterior neck for identification of free unlabeled Tc-99m pertechnetate in the thyroid and salivary glands. Static images of the lateral pelvis can be obtained to help differentiate Tc-99m RBCs in the bladder from that in the rectum.
Criteria for interpretation of a positive RBC scintigraphy scan include demonstration both of extravasation of radiotracer from the vascular compartment and movement of radiotracer in an anterograde and/or retrograde fashion in the bowel lumen.6 Extravasated blood exerts a cathartic effect stimulating Tc99m labeled RBC transit in the bowel. Dynamic imaging minimizes timing errors encountered with static images which may show blood in the gut that has already traveled distal to the actual site of bleeding.10
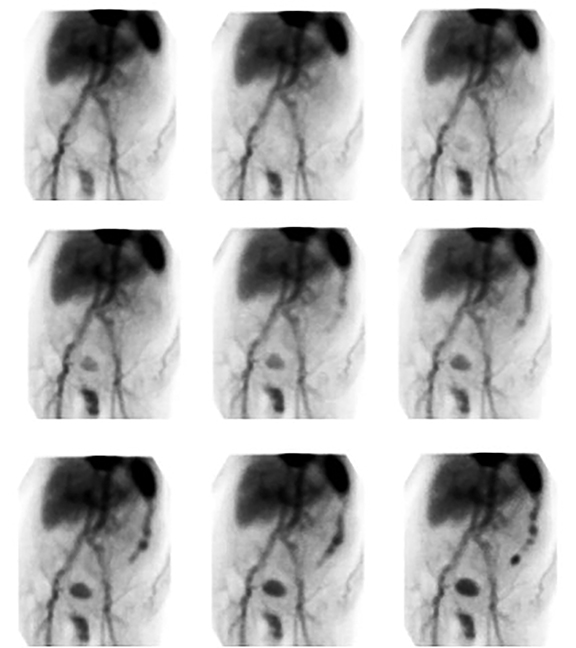
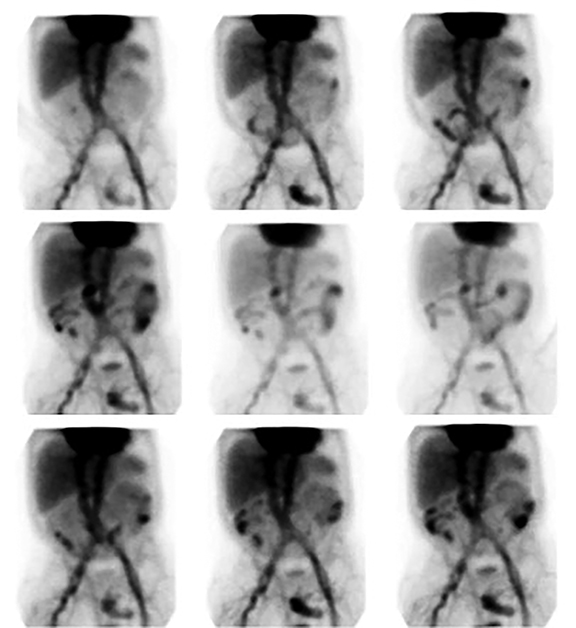
An elongated peripherally localized pattern of radiotracer progression is seen in large bowel GIB (Figure 1). In cinematic display mode, small bowel GIB is distinguishable by demonstration of relatively rapid radiotracer transit through more centrally located curvilinear segments (Figure 2).
False-positive results can be the result of aneurysms, varices, inflammation or tumors and usually are seen as static areas of extravascular radiotracer accumulation on RBC scintigraphy. Penile activity can be mistaken for rectal bleeding, avoided by obtaining lateral pelvic views and positional manipulations. Poor labeling can lead to false positive studies due to free pertechnetate being secreted within gastric mucosa and duodenum and excreted into the urinary collecting system.12 Gallbladder visualization may be due to hematobilia or alternatively transfusion-related labeling of the porphyrin group of degraded hemoglobin, with subsequent liver and biliary excretion, particularly in patients with severe renal impairment.10
Advances
A major limitation of RBC scintigraphy is that it may not provide adequate information for unequivocal localization of the site of GIB. Subtraction scintigraphy when used in conjunction with conventional RBC imaging has been shown to improve diagnostic utility by removal of background superimposed on a bleed; improving image contrast and potentially reducing false-positive studies.13 Combining functional SPECT data with CT (or MRI) via software co-registration of images or image acquisition on dedicated SPECT/CT instrumentation can increase contrast resolution by 10-15% facilitating bleeding source localization by providing accurate anatomical information about the site of 99mTc RBC accumulation.14 However, software fusion of images acquired separately with different devices can lead to inaccuracies in image registration. Imaging with integrated SPECT/CT instrumentation is limited by the time needed to acquire tomographic images and by constraints imposed by the external dimensions of accessory equipment (e.g., ventilators, etc.).
Comparisons
In the emergency setting, RBC scintigraphy plays a complementary role to endoscopy and angiography and should be the initial imaging study in the evaluation of lower GIBs. Colonoscopy, often the initial step in attempting to identify the source of lower GIBs after upper GIB sources have been ruled out, suffers from the potential 4-6 hour delay needed for colon preparation, the need for sedation, the invasive nature of the procedure, the difficulty in identifying bleeding sites during active bleeding, the delay it causes, preventing prompt GIB scintigraphy, and rare but serious complications such as perforation and hemorrhage.4 If colonoscopy is unable to accurately localize the source of bleeding while confirming its presence in the colon, localization with RBC scintigraphy in combination with angiography is often utilized prior to surgical intervention.
Angiographic evaluation of patients with acute GIB ideally involves selective catheterization of the most likely arterial bleeding source as suggested by prior imaging studies such as RBC scintigraphy.15 RBC scintigraphy often plays a significant role in determining both the site of initial catheter placement at angiography and in directing the surgical approach, sometimes in an emergent setting.2 RBC scintigraphy is often performed with the aim of determining a sufficient rate of bleeding to facilitate successful angiography and possible angiographic intervention.10 A positive RBC scintigraphy study increases the likelihood of a positive angiogram from 22% to 53%.
While angiography has the advantage of allowing therapeutic intervention, it has a 9.3% visceral angiography complication rate including acute renal failure, contrast reactions, arterial thrombosis or dissection and bowel infarction.16 Although the specificity of angiography approaches 100% the sensitivity for acute bleeds is 46% and 30% for recurrent, intermittent bleeds. Among the advantages of RBC scintigraphy vs. angiography are the 10-fold greater sensitivity for detection of slow bleeding rates or chronic bleeding, the ability to examine the entire lower GI tract simultaneously and continuously over an extended period of time (60 to 90 minutes), and the ability for repeat imaging out to 24 hours. Many angiographers prefer to have RBC scintigraphy prior to the contrast study to ensure that bleeding is active, i.e., the contrast study is more likely to identify the bleeding site if scintigraphy is positive.
RBC scintigraphy also plays an important role in the diagnosis of small intestinal bleeding, when conventional endoscopy (EGD, push enteroscopy and colonoscopy) has limited value, and costly innovative methods such as capsule endoscopy and double-balloon enteroscopy are not readily available.17 Of note, unlike RBC scintigraphy, capsule endoscopy is contraindicated in patients with known or suspected gastrointestinal obstruction, strictures or fistulae, which can necessitate surgical removal, as well as prior pelvic or abdominal surgery, pregnancy, implanted electronic devices such as pacemakers and extensive Crohn and diverticular disease.16
Conclusion
RBC scintigraphy plays a complementary role to colonoscopy and angiography in the evaluation of lower GIB and should be utilized prior to those more invasive procedures, which often fail to localize and control lower GIB due to intermittent bleeding. The fact that radionuclide imaging is noninvasive, safe and can detect slower bleeding rates than other conventional imaging methods makes it useful in hemodynamically stable patients who are suspected of having intermittent or low rates of GI hemorrhage.18 In addition, RBC scintigraphy helps to stratify high-risk patients who would most benefit from aggressive intervention from those who can be managed medically.
References
- Burke SJ, Golzarian J, Weldon D, Sun S. Nonvariceal upper gastrointestinal bleeding. Eur Radiol. 2007;17(7):1714-1726. doi:10.1007/s00330-006-0477-x.
- Fearnhead NS. Acute lower gastrointestinal bleeding. Medicine (Baltimore). 2007;35(3):164-167. doi:10.1016/j.mpmed.2006.12.008.
- Mariani G, Pauwels EKJ, AlSharif A, et al. Radionuclide evaluation of the lower gastrointestinal tract. J Nucl Med Off Publ Soc Nucl Med. 2008;49(5):776-787. doi:10.2967/jnumed.107.040113.
- Currie GM, Kiat H, Wheat JM. Scintigraphic evaluation of acute lower gastrointestinal hemorrhage: current status and future directions. J Clin Gastroenterol. 2011;45(2):92-99. doi:10.1097/MCG.0b013e3181f39d46.
- Wilkins T, Baird C, Pearson AN, Schade RR. Diverticular bleeding. Am Fam Physician. 2009;80(9):977-983.
- Allen TW, Tulchinsky M. Nuclear medicine tests for acute gastrointestinal conditions. Semin Nucl Med. 2013;43(2):88-101. doi:10.1053/j.semnuclmed.2012.11.001.
- Anthony S, Milburn S, Uberoi R. Multi-detector CT: review of its use in acute GI haemorrhage. Clin Radiol. 2007;62(10):938-949. doi:10.1016/j.crad.2007.02.019.
- Mellinger JD, Bittner JG, Edwards MA, Bates W, Williams HT. Imaging of gastrointestinal bleeding. Surg Clin North Am. 2011;91(1):93-108. doi:10.1016/j.suc.2010.10.014.
- Middleton ML, Strober MD. Planar scintigraphic imaging of the gastrointestinal tract in clinical practice. Semin Nucl Med. 2012;42(1):33-40. doi:10.1053/j.semnuclmed.2011.07.006.
- Howarth DM. The role of nuclear medicine in the detection of acute gastrointestinal bleeding. Semin Nucl Med. 2006;36(2):133-146. doi:10.1053/j.semnuclmed.2005.11.001.
- Ziessman HA, O’Malley JP, Thrall JH, Fahey FH. Nuclear Medicine. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2014.
- Strate LL, Naumann CR. The role of colonoscopy and radiological procedures in the management of acute lower intestinal bleeding. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2010;8(4):333-343; quiz e44. doi:10.1016/j.cgh.2009.12.017.
- Warrington JC, Charron M. Pediatric gastrointestinal nuclear medicine. Semin Nucl Med. 2007;37(4):269-285. doi:10.1053/j.semnuclmed.2007.02.005.
- Currie GM, Towers PA, Wheat JM. Improved detection and localization of lower gastrointestinal hemorrhage using subtraction scintigraphy: clinical evaluation. J Nucl Med Technol. 2007;35(2):105-111. doi:10.2967/jnmt.106.037044.
- Currie GM. Cost-effectiveness analysis of subtraction scintigraphy in patients with acute lower gastrointestinal tract hemorrhage. J Nucl Med Technol. 2007;35(3):140-147. doi:10.2967/jnmt.106.037655.
- Schillaci O, Spanu A, Tagliabue L, et al. SPECT/CT with a hybrid imaging system in the study of lower gastrointestinal bleeding with technetium-99m red blood cells. Q J Nucl Med Mol Imaging Off Publ Ital Assoc Nucl Med AIMN Int Assoc Radiopharmacol IAR Sect Soc Radiopharm Chem Biol. 2009;53(3):281-289.
- Tabibian JH. Technetium-99m-labeled RBC scintigraphy: less relative utility now than ever? J Clin Gastroenterol. 2011;45(7):651-652; author reply 652-654. doi:10.1097/MCG.0b013e31821f8d76.
- Padia SA, Bybel B, Newman JS. Radiologic diagnosis and management of acute lower gastrointestinal bleeding. Cleve Clin J Med. 2007;74(6):417-420.
