Uncommon mixed and sclerotic jaw lesions
By Sureka J, Simon B, Sudhakar S, Masih D
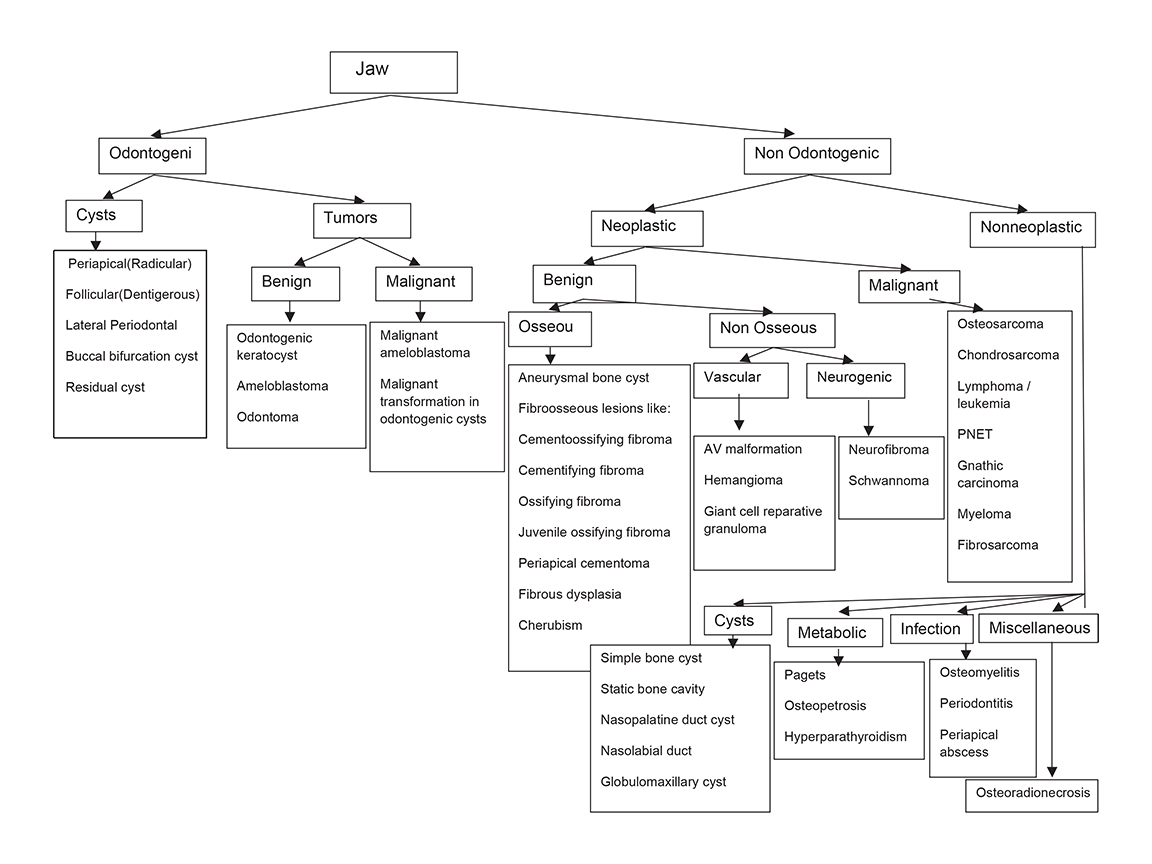




A variety of benign and malignant conditions can affect the jaws, the maxilla and mandible. The similar image appearance of many pathologies can make achieving a single diagnosis difficult in many situations.1 The age of the patient, location of the lesion, relationship to the tooth and adjacent structures, margins of the lesion, internal characteristics such as the pattern of mineralization, periosteal reaction, associated soft tissue changes and relevant clinical details, all help to narrow the differential diagnosis.1 Plain radiography retains its role as the initial mode of evaluation of these lesions.2 Cross-sectional imaging, such as CT and MRI, are useful in tissue characterization, assessing extent of extra-osseous soft tissue and bony involvement in terms of cortical expansion, resorption or destruction, osteosclerosis, and calcification; thus, they help in preoperative evaluation. MRI is superior to CT in differentiating cystic from solid lesions, and in defining the extent of soft tissue and bone marrow infiltration in aggressive jaw lesions. The classification of jaw lesions is summarized in Figure 1.
Radiologic findings of some less common jaw lesions
Odontogetic keratocyst (OKC) or keratocystic odontogenic tumor with dentinoid formation
In 2005, the World Health Organization (WHO) reclassified OKC to highlight the aggressive nature and high recurrence rate of this lesion. It is a benign, locally aggressive tumor of odontogenic origin, with a characteristic lining of parakeratinized stratified squamous epithelium.3 It is typically seen in young patients and commonly located in either the body or the ramus of the mandible.1,4 On CT, these tumors are seen as cystic structures with cortical expansion without solid components. They can be associated with an impacted tooth, mimicking a dentigerous cyst, and can sometimes be complex with multiple septations, making it difficult to differentiat OKC from ameloblastoma.
Multiple lesions are associated with Gorlin-Goltz syndrome or nevoid basal cell carcinoma syndrome. MR imaging can help differentiate OKC and ameloblastoma based on T2 relaxation time, which is more heterogeneous and short in OKC (contents appear more hypointense on T2-weighted images due to keratin), compared to ameloblastoma, where it is more homogeneous and hyperintense.5,6 Other MRI features, such as a thin wall with poor enhancement, are seen in OKC, compared to the thick, enhancing wall and solid component in ameloblastoma.5 Deposition of hard tissue like dystrophic calcification or cartilage in the cyst wall of OKC is rare, and the presence of dentin-like tissue (Figure 2), even more rare,7 can mimic other dentinoid-forming odontogenic cysts and tumors like calcifying epithelial odontogenic tumor, odontoma, ameloblastic fibroodontoma and adenomatoid odontogenic tumor.7
Odontoma
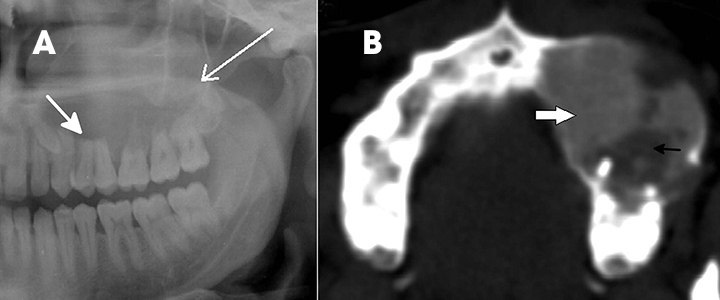
Odontomas are considered to be “tooth hamartomas” rather than true neoplasms. They appear in children as pericoronal sclerotic lesions with a thin radiolucent rim and are commonly associated with an impacted tooth. Odontomas can be simple (supernumerary teeth), compound or complex.1,2,8 Compound odontomas are composed of several well-formed, tooth-like structures (Figure 3A), while complex odontomas show irregular, amorphous hyperattenuating, calcified tissue surrounded by a radiolucency (Figure 3B). Odontomas can be differentiated from osteomas by the thin radiolucent line that surrounds them.9
Ameloblastic fibro-ondotoma
Ameloblastic odontomas can be classified into ameloblastic fibro-odontoma and odonto-ameloblastoma. Odonto-ameloblastoma is an ameloblastoma containing mineralized dental tissue. Ameloblastic fibro-odontoma is classified as a benign, mixed-odontogenic neoplasm containing neoplastic epithelium and mesenchyme. It is common in a younger (less than 20 years) age group and consists of variable amounts of components of the developing teeth, ranging from homogenous mass-like structures to loose calcified strands radiating from the center to the periphery of lytic lesions, giving the appearance of wheel spokes.1,2,8,10 (Figure 4).
Odontogenic myxoma
Odontogenic myxoma is a locally aggressive, benign tumor with variable radiographic appearances.1,2,4,11 It is commonly seen as a multiloculated, radiolucent lesion with internal osseous trabeculae and involving the tooth-bearing area. Varying radiographic appearances have been divided into 6 types as follows: unilocular, multilocular (including honeycomb, soap bubble and tennis racquet patterns), involvement of local alveolar bone, involvement of the maxillary sinus, osteolytic destruction and a mix of osteolytic destruction and osteogenesis (Figure 5).12 When features are not classical, the tumor can mimic ameloblastoma, Pindborg tumor, fibrous dysplasia, ossifying fibroma and even osteosarcoma when the tumor perforates the cortex with the formation of multiple, radiating spicules giving a sunburst appearance.12
Calcifying epithelial odontogenic tumor or Pindborg tumor
Pindborg tumor consists of epithelial tissue within a fibrous stroma. It is also associated with an unerupted or impacted tooth and is commonly seen around the mandibular premolar or molar teeth as a radiolucent lesion with variable calcification (Figure 6).1,2,4,11,13
Adenomatoid odontogenic tumor
This is an uncommon, slow-growing, hamartomatous and benign epithelial lesion of odontogenic origin that is frequently misdiagnosed on radiographs as other odontogenic cysts and tumors. Unlike many other cystic lesions of the jaws, these lesions occur more commonly in the maxilla than in the mandible. On radiographs they appear as well-defined expansile lytic lesions with variable internal calcifications.
Like dentigerous cysts, they are associated with impacted teeth; however, these tumors usually extend beyond the cemento-enamel junction and include the tooth root with variable calcification around the embedded tooth (Figure 7).1,2,4,11,8,13
Benign fibro-osseous lesions: Ossifying fibroma, cementifying fibroma and cemento-ossifying fibroma
Benign fibro-osseous lesions of the jaw include a spectrum of developmental, reactive or neoplastic lesions characterized by replacement of normal bone with fibrous tissue that, over time, is infiltrated by osteoid or cementoid tissue. These lesions are believed to arise from cells of the periodontal membrane. They are broadly classified as fibrous dysplasia and ossifying fibroma. Ossifying fibromas include cementifying fibromas, cemento-ossifying fibromas and juvenile ossifying fibromas. Psammomatoid and trabecular types of juveline ossifying fibromas are recognized.9 Dysplastic conditions around the teeth, such as periapical, focal or florid cementoosseous dysplasias, are also included in some classifications like that proposed by Charles A Waldron.14 Depending on their evolution, these lesions can vary in appearance, with a more lucent appearance seen initially due to predominant fibrous tissue and deposition of dense osteoid or cementum-like material.9 Cementifying fibromas (Figure 8) and cemento-ossifying fibroma are very similar to ossifying fibromas, but they have a greater tendency to form cementum-like material that is more ovoid and heavily calcified. However, in ossifying fibroma, the calcifications are more spiculated, with a ground-glass appearance (Figure 9). Ossifying fibromas show a narrow transition zone from surrounding normal bone, thus differentiating them from fibrous dysplasias. Another differentiating feature is displacement of teeth in ossifying fibroma, unlike fibrous dysplasias.9
Cemento-osseous dysplasia/periapical osseous dysplasia
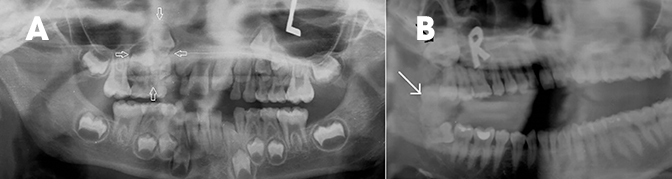
The 2005 WHO classification divides these bone-related lesions into periapical, focal, florid cemento-osseous dysplasias and familial gigantiform cementomas.3 These appear as single or multifocal, well-defined, cement-like sclerotic lesions in a periapical location with surrounding lucent halo (Figure 10). Middle-aged women are most commonly affected. The closest differential is cementoblastoma (Figure 11), which occurs in children and fuses to the tooth root, unlike cementosseous dysplasia. Like ossifying fibromas, these lesions also show temporal evolution in appearance from early lucent lesions to progressive increase in density. Early lucent lesions can mimic periapical inflammatory lesions; however, the latter is unlikely in a normal tooth with no caries or previous treatment.9
Fibrous dysplasia — cherubism
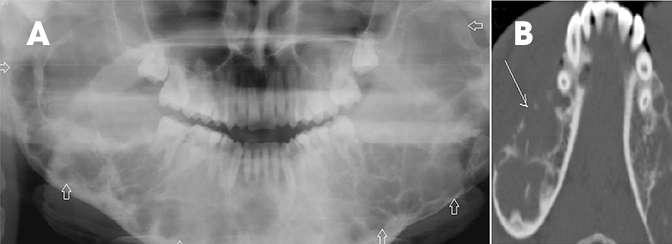
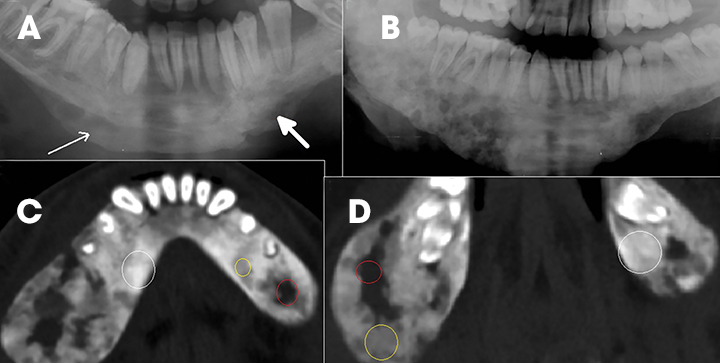
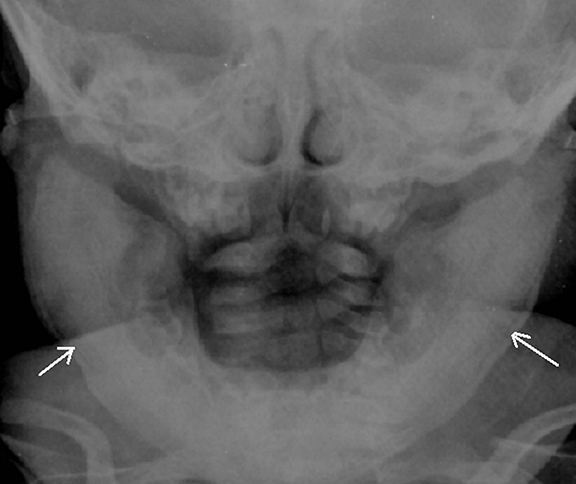
Fibrous dysplasia is a disturbance of bone metabolism where the fibrous connective tissue containing abnormal bone replaces normal bone. It can exist in monostotic and polyostotic forms.2 In its typical form it has ground glass matrix however, atypical forms can mimic other fibro-osseous lesions like ossifying fibroma in the craniofacial region. A rare variant of this entity called Cherubism is a familial form affecting children and adolescents and typically involving only the maxilla and mandible. 2,15 On imaging it is seen as expansile remodeling of the maxilla and mandible with thinning of the cortices, multilocular radiolucencies and a coarse trabecular pattern (Figure 12).
Benign bone and cartilage-forming tumors—multiple osteomas in Gardner’s syndrome
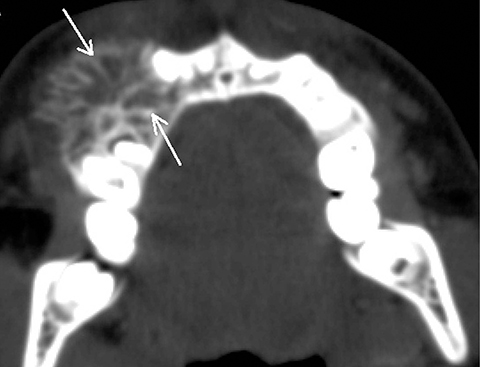
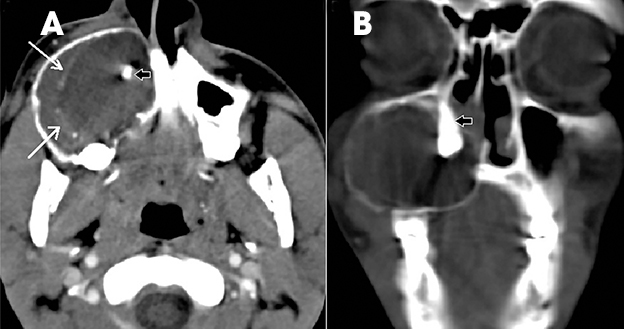
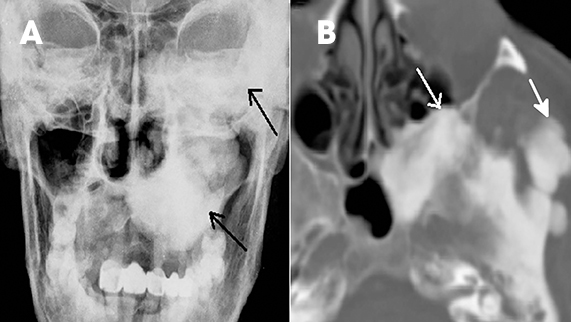
Osteomas are slow-growing, benign, bone-forming tumors commonly occurring in the craniofacial bones and characterized by the proliferation of compact and/or cancellous bone. In the jaws, they occur along the lingual side of the ramus or the inferior mandibular border below the molars. On imaging, an osteoma appears as a well-defined, non-tooth related, dense and radiopaque mass with no surrounding radiolucent line, with or without bone expansion. In the absence of expansion, differentiation from idiopathic osteosclerosis may be difficult.9 Multiple carniofacial osteomas, along with cutaneous and soft-tissue tumors, can suggest a diagnosis of Gardner’s syndrome 2 (Figure 13).
Vascular lesions
Arteriovenous malformation/hemangioma
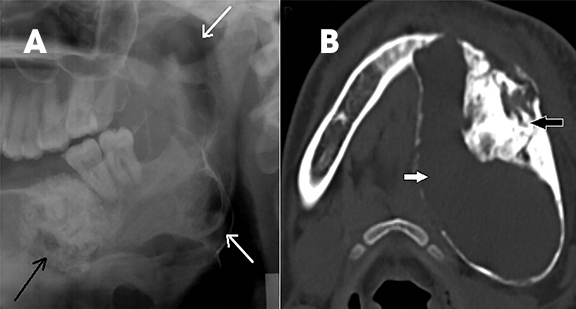
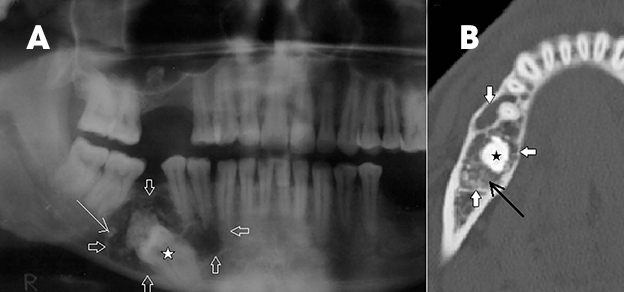
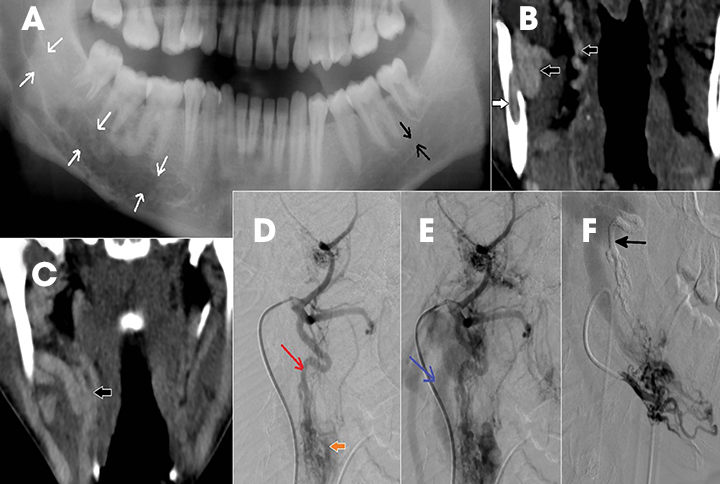
Intraosseous AVM is rare and recognition is important because extraction of an associated tooth may lead to fatal hemorrhage.2,4 In the jaw, they usually occur within the ramus and posterior mandibular body.1 Radiographically, they appear as multilocular cystic lesions, and angiography is usually necessary to demonstrate the vascular nature of the lesion (Figure 14).2 Angiography clearly demonstrates the feeding and draining vessels and also helps in treatment planning.
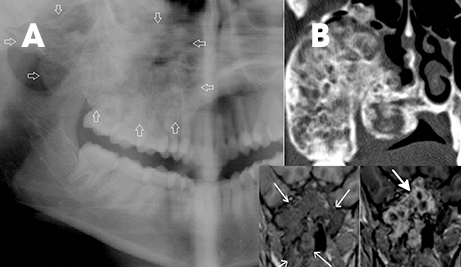
The intraosseous or central hemangioma is frequently found in the vertebrae and skull and rarely seen in the jaws.16 These lesions are also great mimickers, with different radiographic appearances. Most commonly, lesions show a multilocular radiolucency with small (honey-comb appearance) or large (soap-bubble appearance) loculations. Phleboliths or vascular calcifications may also appear as rounded or oval radioopacities.16 They can mimic ameloblastoma, keratocyst and fibro-osseous lesions such as fibrous dysplasia when they have honeycomb or soap-bubble appearances 17 (Figure 15). CT and MRI allow clear identification of the lesion’s vascular nature and also can delineate the extent of involvement. Honeycombed appearance and periosteal reaction are described as rare CT findings.17
Infective and inflammatory conditions
Osteomyelitis
Periodontitis, periapical abscess and osteomyelitis are important entities in this group of infective/inflammatory conditions. Apical periodontitis includes a spectrum of periapical cysts, granulomas and abscesses. They are mostly related to periodontal diseases,2 such as dental caries and irreversible pulpitis. Distinguishing apical cysts and granulomas on imaging is difficult. Thickening of the periodontal ligament space can be the earliest sign. If apical abscess forms, the enhancing rim can be seen on images. Progression of the infection to osteomyelitis or inflammation of the bone and bone marrow is rare due to the early administration of antibiotics. Acute osteomyelitis may not demonstrate any changes on plain radiography, while chronic osteomyelitis may appear as focal or diffuse lytic or sclerotic areas with periosteal reaction.18 All three patterns of CT findings in bone in chronic osteomyelitis are illustrated in Figure 16.
Systemic disease affecting the jaws
Systemic diseases that can commonly affect the jaws include Paget’s disease, osteopetrosis, Caffey’s disease, eosinophilic granuloma, brown tumor of hyperparathyroidism and, rarely, amyloid deposits. Associated changes in other bones, clinical features and biochemical parameters will help complete the final diagnosis in these systemic conditions.2
Caffey disease
Caffey disease, or Infantile Cortical Hyperostosis, is a rare, self=limiting disorder of unknown etiology and affecting infants, usually younger than 6 months of age. It is characterized by inflammation of the periosteum and the overlying soft tissue along with systemic changes of irritability and fever. The mandible, ribs, clavicles, scapula and long bones can be involved. Radiographs show classical cortical hyperostosis beneath regions of soft-tissue swelling 19 (Figure 17). Osteomyelitis, chronic hypervitaminosis A, scurvy and child abuse are common differentials.
Hyperparathyroidism
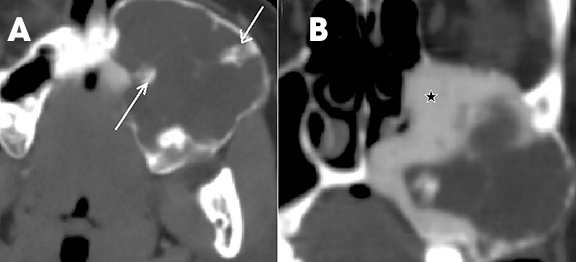
Brown tumors are non-neoplastic lesions found in hyperparathyroidism and can involve the maxilla or mandible.11 They arise from a change in bone metabolism and present clinically and radiologically as expansile masses. One or more lesions may be seen in a bone. They have variable wellor ill-defined margins (Figure 18). Associated changes, such as bone density, granular texture of bony trabeculae, and loss of the lamina dura in other teeth, can point towards hyperparathyroidism. They are very similar histologically and radiologically to other giant-cell lesions and differential diagnosis includes giantcell reparative granuloma, true giant-cell tumor and aneurysmal bone cyst. The familial type of hyperparathyroidism with associated lesions in the jaw and kidney is called hyperparathyroidism–jaw tumor (HPT-JT) syndrome. The jaw lesions are usually fibro-osseous and the renal involvement may be either as cysts or as tumors, such as Wilm’s tumor.20
Conclusion
Jaw lesions are fairly common in radiology practice. Familiarizing oneself with different conditions and their appearance on imaging can help radiologists to reach a reasonable differential diagnosis.
REFERENCES
- Dunfee BL, Sakai O, Pistey R, et al. Radiologic and pathologic characteristics of benign and malignant lesions of the mandible. RadioGraphics. 2006;26(6):1751-1768.
- Neyaz Z, Gadodia A, Gamanagatti S, et al. Radiographical approach to jaw lesions. Singapore Med J. 2008;49(2):165-176.
- Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and Genetics Head and Neck Tumours. Lyon: IARC Press; 2005.
- Meyer KA, Bancroft LW, Dietrich TJ, et al. Imaging characteristics of benign, malignant, and infectious jaw lesions: A pictorial review. AJR Am J Roentgenol. 2011;197(3) : W412-421.
- Minami M, Kaneda T, Ozawa K, et al. Cystic lesions of the maxillomandibular region: MR imaging distinction of odontogenic keratocysts and ameloblastomas from other cysts.AJR Am J Roentgenol. 1996;166(4):943-949.
- Minami M, Kaneda T, Ozawa K, et al. Ameloblastoma in the maxillomandibular region: MR imaging. Radiology. 1992; 184:389-393.
- Ng KH, Siar CH. Odontogenic keratocyst with dentinoid formation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 95:601-606.
- Yonetsu K, Nakamura T. CT of calcifying jaw bone diseases. AJR Am J Roentgenol. 2001; 177:937-943.
- Curé JK, Vattoth S, Shah R. Radiopaque jaw lesions: An approach to the differential diagnosis. Radiographics. 2012;32(7):1909-1925.
- Gyulai-Gaál S, Takács D, Szabó G, et al. Mixed odontogenic tumors in children and adolescents. J Craniofac Surg. 2007;18(6): 1338-1342.
- Scholl RJ, Kellett HM, Neumann DP, et al. Cysts and cystic lesions of the mandible: Clinical and radiologic-histopathologic review. RadioGraphics. 1999;19(5):1107-1124.
- Zhang J, Wang H, He X, et al. Radiographic examination of 41 cases of odontogenic myxomas on the basis of conventional radiographs. Dentomaxillofac Radiol. 2007;36(3):160-167.
- Butt FM, Ogeng’o J, Bahra J, et al. Pattern of odontogenic and nonodontogenic cysts. J Craniofac Surg. 2011; 22(6):2160-2162.
- Waldron CA. Fibroosseous lesions of the jaws. J Oral Maxillofac Surg. 1985; 43(4):249-262.
- Beaman FD, Bancroft LW, Peterson JJ, et al. Imaging characteristics of cherubism. AJR Am J Roentgenol. 2004; 182(4):1051-1054.
- Zlotogorski A, Buchner A, Kaffe I, et al. Radiological features of central haemangioma of the jaws. Dentomaxillofac Radiol. 2005;34(5):292-296.
- Gómez Oliveira G, García-Rozado A, Luaces Rey R. Intraosseous mandibular hemangioma. A case report and review of the literature. Med Oral Patol Oral Cir Bucal. 2008;13(8):E496-498.
- Tanaka R, Hayashi T. Computed tomography findings of chronic osteomyelitis involving the mandible: Correlation to histopathological findings. Dentomaxillofac Radiol. 2008; 37(2):94-103.
- Restrepo S, Sánchez AM, Palacios E. Infantile cortical hyperostosis of the mandible. Ear Nose Throat J. 2004; 83(7):454-455.
- Carlson AL, Smith CL. Primary hyperparathyroidism and jaw tumor syndrome: A novel mutation of the HRPT2 gene. Endocr Pract. 2008;14(6):743-747.
