18F-FDG PET/CT for cardiac implantable electronic device infection
Images
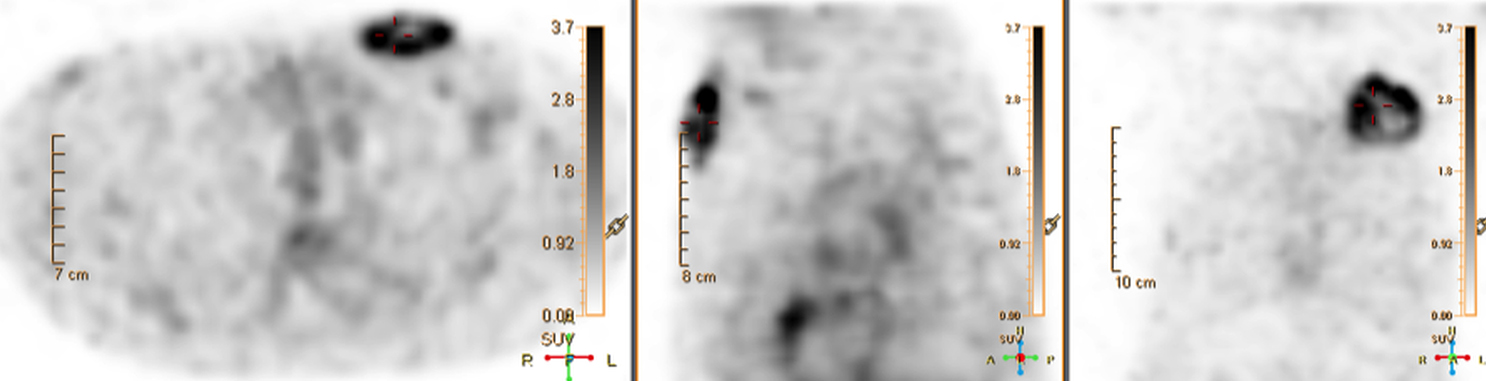
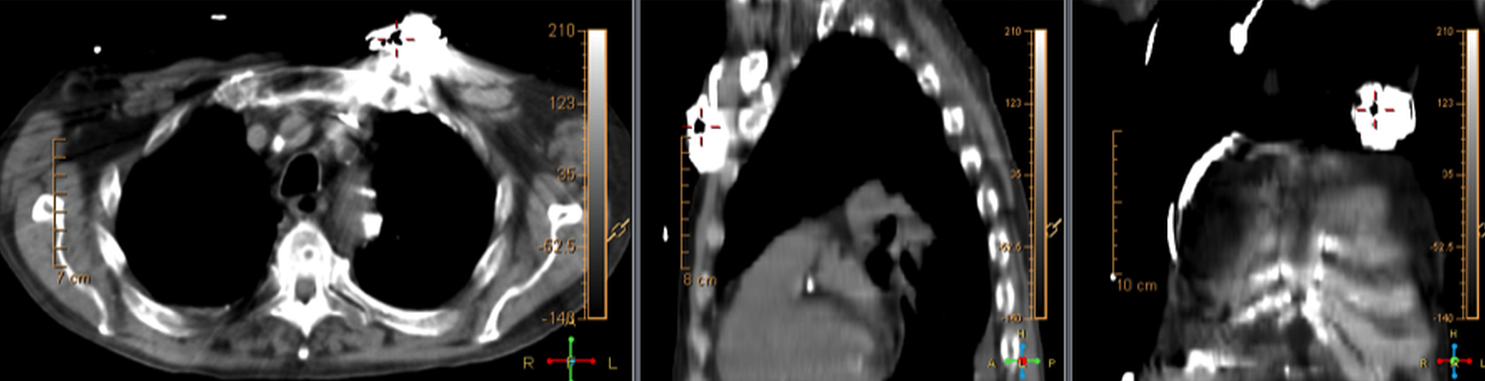
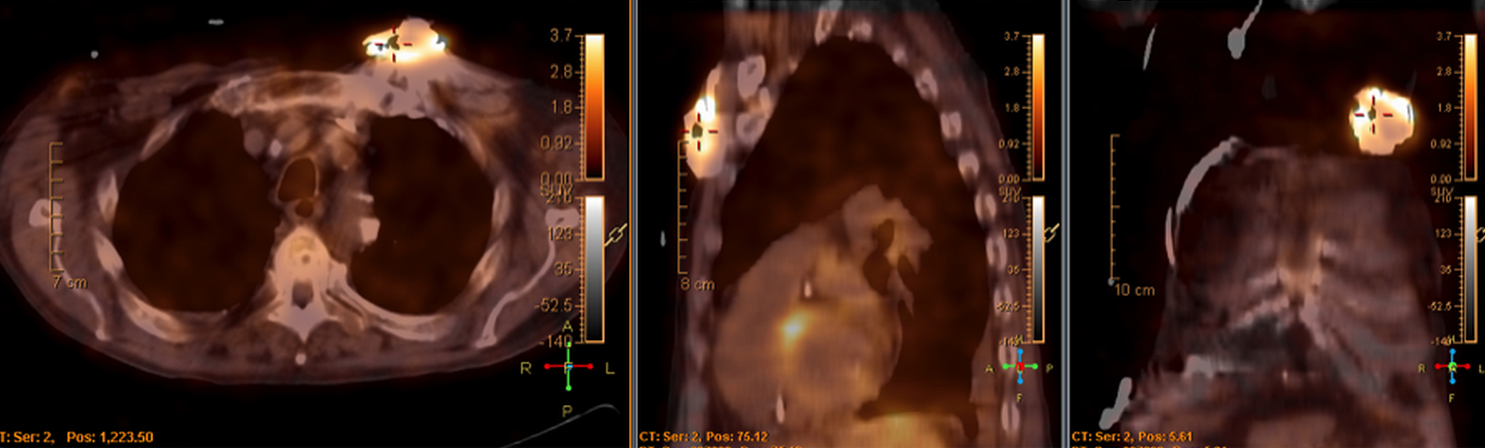




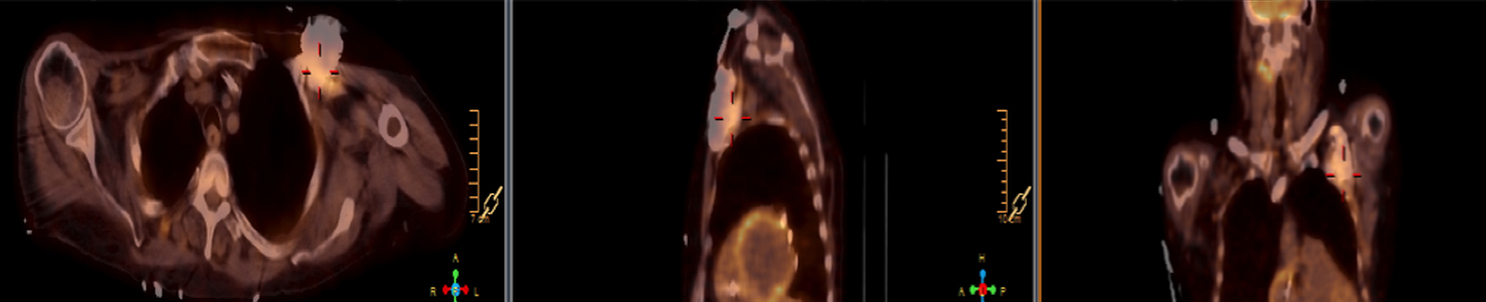
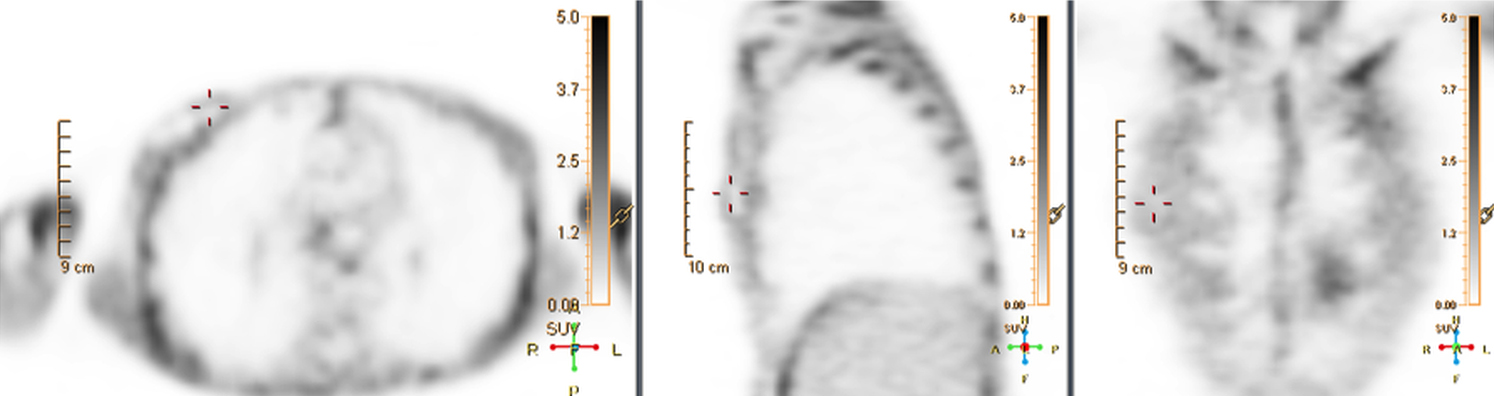
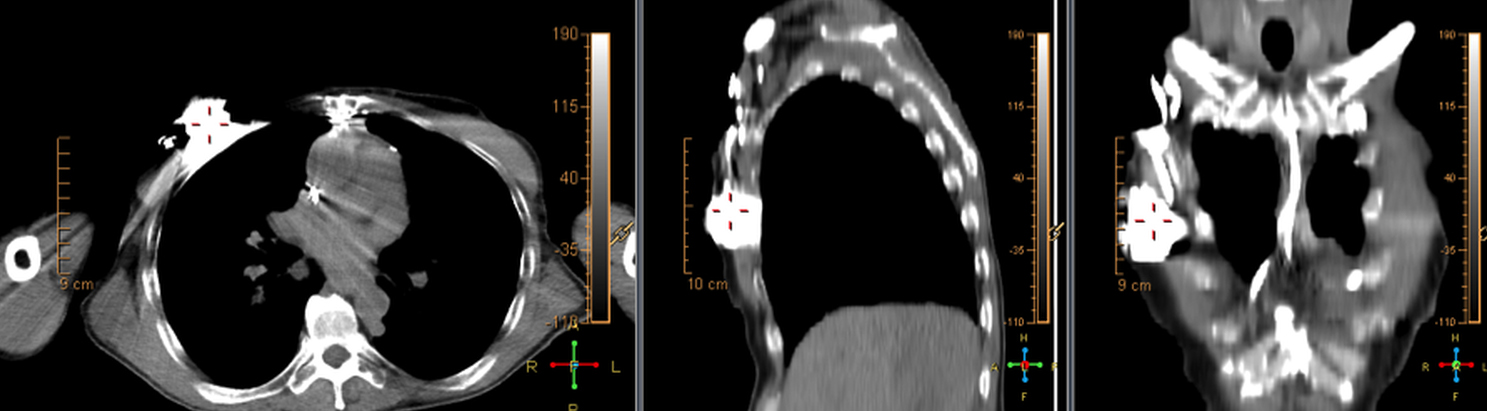
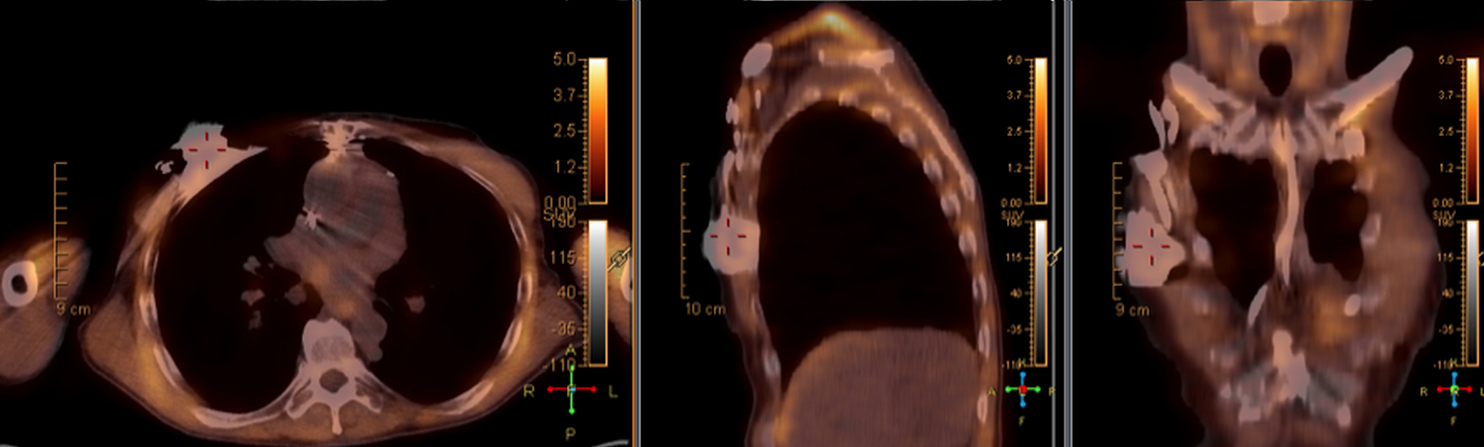
There are many challenging infectious disease questions in diagnostic radiology where18F-FDG PET/CT may be a practical and useful solution.1,2 One of them is related specifically to infection of cardiac implantable electronic devices (CIED). There are an increasing number of CIED procedures accompanied by an even higher rate of increasing cases of infection, partially due to the aging of the new device recipients with multiple comorbidities and longer hospital stays.3 Infection of a foreign body such as a CIED can quickly present a high risk of mortality, if not treated appropriately. There is an increasing need for diagnostic evaluation of a CIED which remains a challenge for current anatomical imaging modalities. For example, CT and MRI findings are non-specific for infection and are affected by metal artifacts. Transthoracic (TTE) or transesophageal (TEE) echocardiography is limited to evaluation of CIED lead related intra-cardiac vegetation, and not useful for the extra-cardiac portion of the lead and the device pocket.
18F-FDG PET/CT may provide added value in the evaluation of CIED infection in a clinically suspected case, and help to localize the site of infection, for example, whether there is an infectious process involving a CIED deep pocket, or if an infection is simply limited to the superficial tissues, which requires different management. A key advantage of18F-FDG PET/CT over anatomic imaging modalities is detecting infections early, before morphological changes ensue.4 Areas of infection have increased glucose uptake due to increased expression of glucose transporter (GLUT) family isotypes among activated immune cells in areas of infection and bacteria’s reliance on glycolysis as an energy source.5 18F-FDG PET/CT offers similar to relatively higher sensitivity and specificity as other nuclear radiotracers used in infection such as WBC scan, with the additional benefits of safety/ease of use, lower radiation dose, and improved spatial resolution.6 An added advantage of18F-FDG PET/CT is the ability to detect other potential sources of infection in a single study because it is a full body scan.
There are several important challenging management questions involving CIED where18F-FDG PET/CT can be particularly useful. Is the patient likely to respond to IV antibiotics? Will the CIED need to be replaced? Is there an infection along a lead? Is there another unknown source of infection somewhere else that is complicating the clinical picture?
Diagnostic interpretation
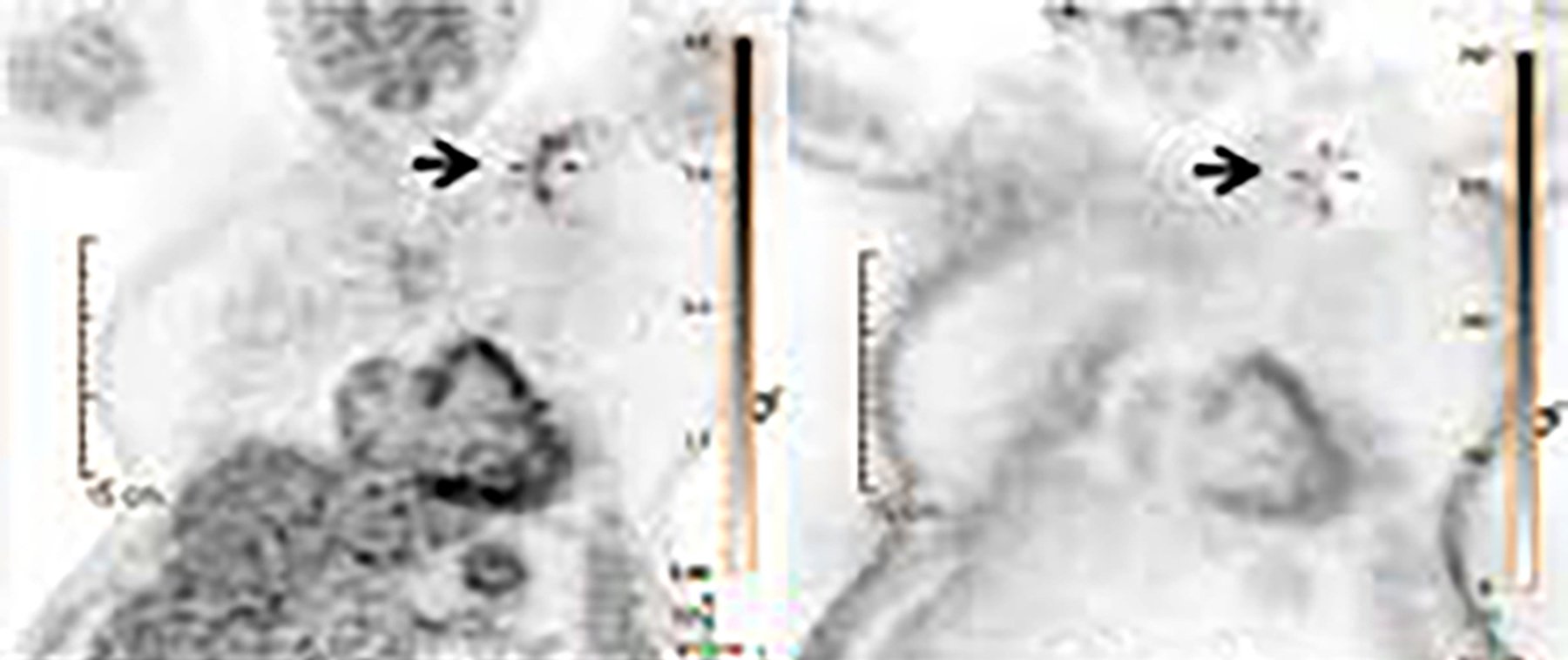
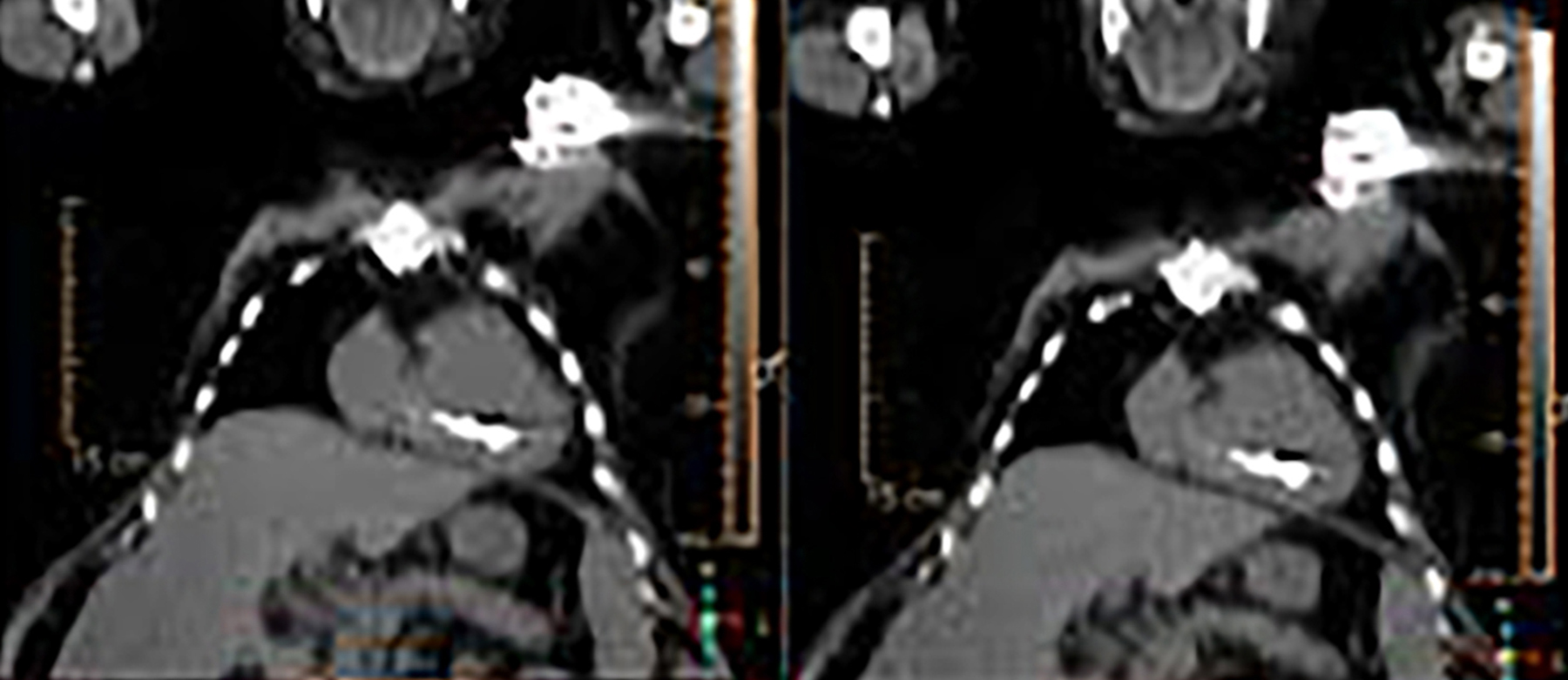
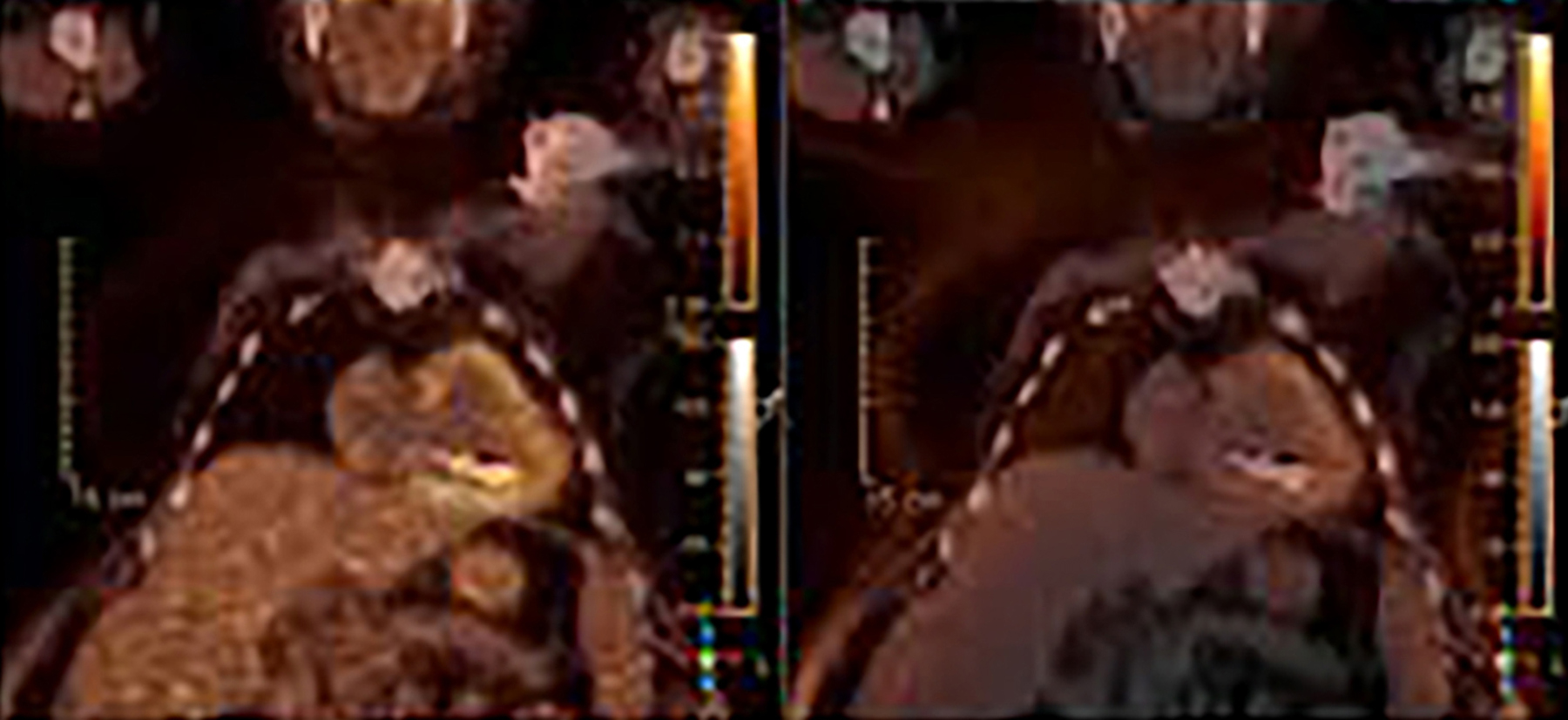
When evaluating CIEDs with18F-FDG PET/CT, there are three primary areas where close attention is necessary. It is important to evaluate the superficial tissues above the device in the chest wall, the device pocket, and the leads. If infection is limited to the superficial tissues, but not in contact with the device itself or the deep tissues, patients typically respond well to treatment for the superficial skin infection alone without the need for device removal. If infection is deeper or involves the device, then device removal is necessary (Figure 1).
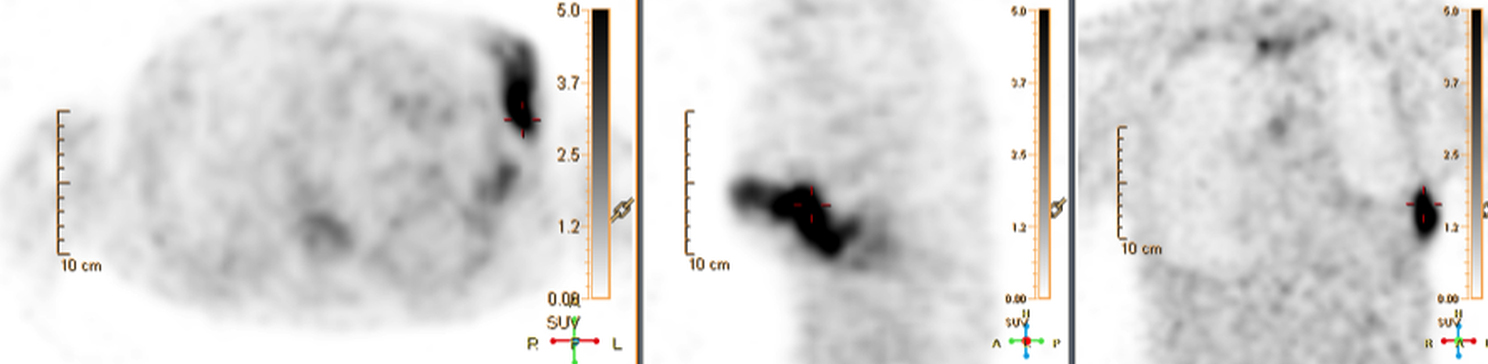
Device lead infection can present a diagnostic challenge because it can often not be appreciated on the surface and is often not associated with anatomical imaging findings such as a fluid collection. The diagnostic clue is focal increased18F-FDG uptake along the lead (Figure 2). Focal increased uptake in a patient with concern for lead infection should prompt complete removal of the device and leads.18F-FDG PET/CT may not be able to easily detect an intra-cardiac vegetation at the tip of the lead given the small size and also moving vegetation in a background of physiologic cardiac18F-FDG activity. On the other hand, vegetation detected on PET indicates an ongoing infection and guarantees entire device removal. Although TTE and TEE can also identify intra-cardiac lead vegetation, they can be chronic or treated—not representative of active infection.7
Infection can present in any of these regions of a CIED with the qualitative appearance of focal increased uptake. Inflammatory change potentially may be differentiated from infection based on a more homogeneous pattern of relatively less increased18F-FDG uptake (Figure 3). Increased signal on18F-FDG PET due to inflammation resolves within 4-8 weeks after implantation of the CIED.6 Negative cases generally show no18F-FDG activity along the devices (Figure 4).
Clinical pitfalls and mimics
Common causes of false negatives are previously treated infections, or sometimes less severe infections. There are reported cases of patients with suspicion for CIED infection and without increased18F-FDG uptake on PET.8 Nevertheless, in negative studies patients demonstrated good outcomes with a course of intravenous antibiotics alone and did not require device removal. These results suggest that18F-FDG PET/CT could have a greater role than diagnosis alone by also helping to guide management decisions. Hyperglycemia is another important cause of false negatives.8
Since18F-FDG PET/CT is primarily utilized to exclude infection or to understand the extent of a suspected infection, in most cases the pre-test probability for infection is high. Therefore, false positives may be less of a concern clinically. Without18F-FDG PET/CT evaluation, clinicians would often have little option except to remove the CIED in a suspected infection.
False positives can be caused by artefactual uptake due to metallic objects when CT attenuation correction is used to post-process the PET data. For this reason, it is suggested that non-attenuation corrected images be evaluated for cases of potential CIED infection in18F-FDG PET/CT (Figure 5). A Dacron pouch can also cause a false positive.8 Thrombus, which can commonly be seen along lead tracts can also demonstrate increased18F-FDG uptake on PET and cause a false positive.9
Conclusion
The qualitative appearance of focal increased uptake is the most reliable way to distinguish infection from inflammation. The latter generally appears homogeneous with low intensity within 4-8 weeks in the case of a recently implanted device.
If there is increased uptake on18F-FDG PET/CT only in the superficial tissues or if the patient is infected clinically, yet demonstrates no increased uptake in the region of the CIED on18F-FDG PET/CT, then the infection can be treated with IV antibiotics without removal of the device. If there is increased uptake within the pocket of the CIED, particularly focal uptake near the device itself or deep to the device, then infection is likely and the device and leads should be removed. Finally, focal increased uptake along a lead wire, but not diffuse mild uptake, is also concerning for lead infection.
Avoid using attenuation-corrected images alone to interpret PET uptake in the setting of CIED infection as this can lead to false positives. Although false negatives occur, they may occur in the setting of less severe infection or treated infection. When infection is suspected clinically, a negative18F-FDG PET/CT study correlates with clinical response to IV antibiotic treatment alone.
The potential ability to spare some patients the risks (improving quality) and decrease the healthcare system cost of unnecessary CIED removal and replacement supports the use of18F-FDG PET/CT for evaluation of suspected CIED infection in the era of value-based care.
References
- O’Doherty MJ, Barrington SF, Campbell M, et al. PET scanning and the human immunodeficiency virus-positive patient. J Nucl Med. 1997;38(10):1575-1583.
- Basu S, Chryssikos T, Moghadam-Kia S, et al. Positron emission tomography as a diagnostic tool in infection: present role and future possibilities. Semin Nucl Med. 2009;39(1):36-51.
- Kurtz SM, Ochoa JA, Lau E, et al. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993-2006. Pacing Clin Electrophysiol. 2010;33(6):705–711.
- Chen W, Kim J, Molchanova-Cook OP, et al. The potential of FDG PET/CT for early diagnosis of cardiac device and prosthetic valve infection before morphologic damages ensue. Curr Cardiol Rep. 2014;16(3):459-463.
- Meller J, Sahlmann CO, Scheel AK.18F-FDG PET and PET/CT in fever of unknown origin. J Nucl Med. 2007;48(1):35-45.
- Love C, Palestro CJ. Radionuclide imaging of infection. J Nucl Med Technol. 2004;32(2):47-57.
- Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Duke Endocarditis Service. Am J Med. 1994;96(3):200–209.
- Sarrazin JF, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol. 2012;59(18):1616-1625.
Citation
M M, W C.18F-FDG PET/CT for cardiac implantable electronic device infection. Appl Radiol. 2017; (6):16-19.
June 8, 2017