MRI Safety: Prepare for New Guidance
By Gilk T
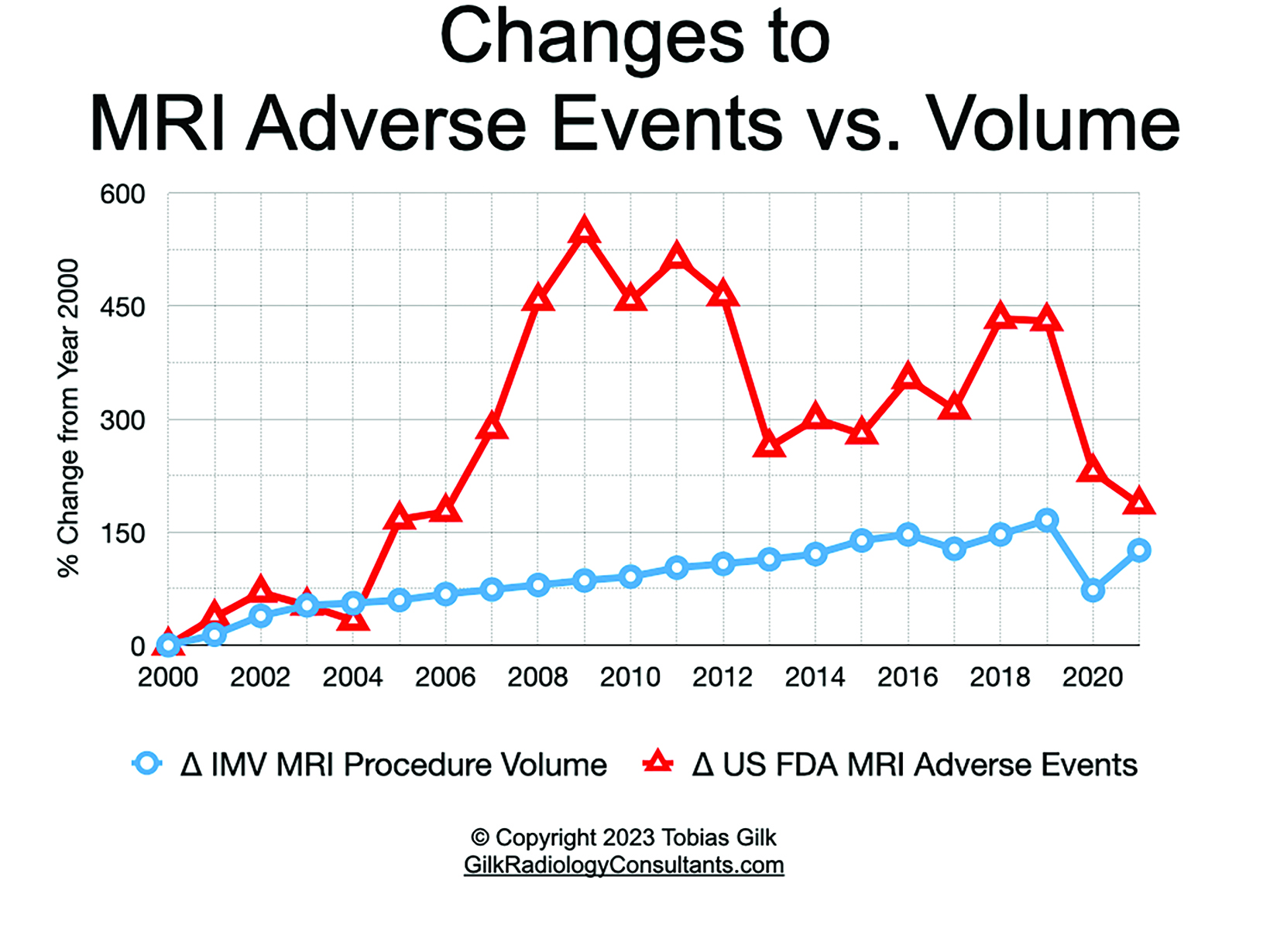
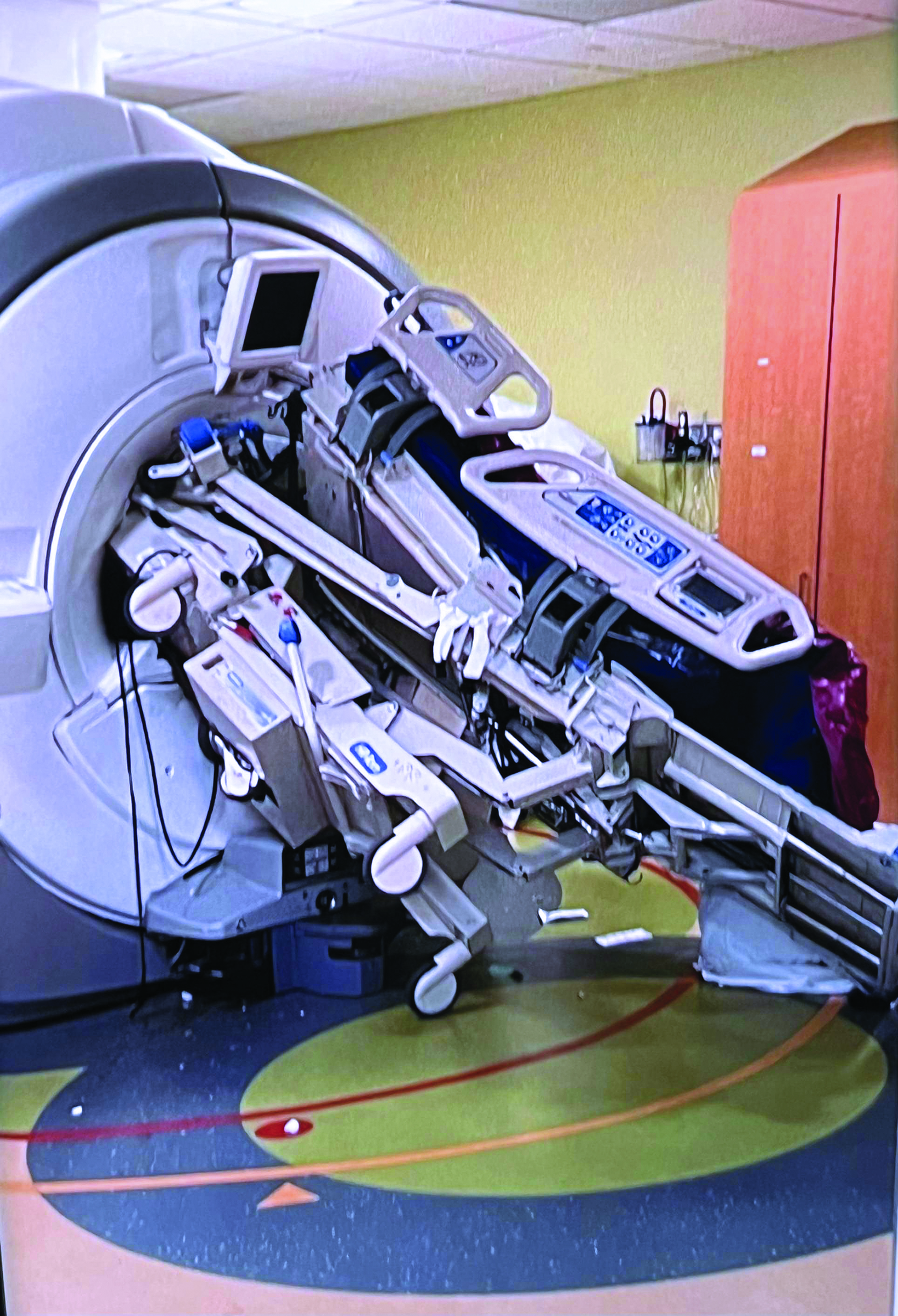
For more than two decades, the American College of Radiology (ACR) has offered a collection of guidance documents outlining a number of best practices to maximize safety in hospital-based and freestanding MRI facilities.
These documents are poised to receive significant expansion under an effort to update the ACR’s 2020 “Manual on MR Safety.”1 Public comment on the proposed changes is now closed, but if implemented in its entirety, the 135-page document will include a wide range of additions.1 These include:
- An introduction to the risks of MRI;
- Expanded information on MRI safety management policies and procedures;
- Recommendations of minimum training criteria for Level 1 and Level 2 safety training;
- An initial safety framework particular to remote-scanning environments;
- Significantly expanded content on risk identification, assessment, and mitigation;
- Guidance for physiologic monitoring of patients;
- Guidance on emergency response practices;
- Discussion of “alternative MRI environments,” including PET/MR, MR-guided Linac devices, and point-of-care systems; and,
- New appendices with checklists for site policies, spatial field gradient data, and conducting risk assessments of implanted devices.
Additionally, the draft document has been organized into chapters to allow for easier navigation, and a number of figures and illustrations have been added to provide examples of risks, harms, and best practices.
One of the most important improvements to the document lies in explaining the ‘why’ of MRI risks and not just enumerating the tasks that prevent them. This expansion of scope consists of a description of risks throughout the document, including the new appendix dedicated to conducting MRI risk assessments. These changes alone represent a shift in the ACR’s intent to transform the document from an “instruction manual” to more of a “teaching tool.”
Since implementing its original MRI safety guidance in 2002, the ACR has long advocated for two distinct levels of training but had not—prior to this draft publication—provided any specific guidance on what each of those levels should contain. Until now, the details of training content and implementation has been determined by the supervising radiologist.
The proposed draft also addresses remote scanning, which is perhaps the most contentious of MRI safety issues. Virtually all contemporary MRI safety guidance (including the ACR’s) is predicated on the notion that the technologist performing the exam is physically present at the point-of-care. Increasingly, however, and similar to how radiologists have had the ability to read electronic studies remotely, new software options from many of the leading MRI manufacturers now allow the operating MRI technologist to be in an entirely different location from where the images are being acquired. With the MRI technologist located remotely (sometimes controlling two or more remote MRI scanners simultaneously), standards of point-of-care workflow and responsibilities have not yet been clearly defined.
The new section on remote scanning seeks to lay out an alternative path for remote scanning safety without entirely rewriting guidance for point-of-care scanning. One of the challenges, however, is the multiplicity of ways that remote scanning can be deployed (eg, technical, teaching, expert model, and full operation). At last year’s RSNA meeting Siemens provided several presentations in their booth about how Advent Health, an integrated healthcare system headquartered in Florida, was deploying remote scanning.
Advent Health’s use of remote scanning includes remote updating of scanner protocols and pulse sequences, “over-the-shoulder” training of remote technologists by senior MR technologists, remote expert scans (eg, a remote cardiac MR expert executing a specialty scan at a remote community hospital or imaging center), and full-shift coverage of remote MR facilities operation during unplanned technologist absences.
The sections of the proposed draft that cover remote scanning are largely dedicated to a discussion of staffing location, with comparatively little discussion devoted to how workflows, decision making, and communications would be required to change under remote scanning.
Owing to the shifting nature of MRI scanning hardware, the draft Manual also features a section on “alternative MR environments.” From portable devices like the Hyperfine Swoop, to dedicated neonatal systems like the Aspect Embrace, to MR-Linacs designed to be retrofitted into existing radiation therapy bunkers, MRI scanners are now found throughout many areas of the hospital besides the radiology department. This necessitates alternative sets of standards as well as careful integration of MRI safety practices within the area(s) where the MRI scanner is operating.
As of this writing the ACR has not offered an anticipated release date for the final document; it is expected to take quite some time for the ACR to consider and decide what additions and/or deletions to make prior to publication. Ultimately, however, the next edition of the ACR Manual on MR Safety can likely be expected to continue defining the standard of care with respect to MR imaging performance and safety.
