Lumbar and cervical pain management procedures: When and how to do them
Images
Dr. Safriel is the lead neuroradiologist in the Dunedin NeuroSpine Institute, University of South Florida, Radiology Associates of Clearwater, Clearwater, FL.
Pain of spinal origin is a significant public health concern in the United States and is among the most common reasons people seek medical care.1–4 It is the also the second leading, medically related reason for missed work, resulting in 40% of lost workdays annually.2 Between 1997 and 2006, the use of interventional pain management procedures increased by 235% in the Medicare population.2 The cost-per-injection also doubled, from $115 to $227, between 1994 and 2001.1 While clinical trials are difficult to perform due to the multiple factors that can influence a patient’s perception of pain, evidence is building that early imaging and intervention result in better outcomes.1–9
Conservative approaches to pain management utilize medication, rehabilitative therapies and behavioral approaches. Interventional pain management approaches employ diagnostic and therapeutic techniques to assist in identifying and relieving pain. Compared to traditional pain management methods, which have shown mixed outcomes,10–12 interventional therapies that rely on injection procedures have demonstrated promising results.5–22
Degenerative back and neck pain is most easily classified into 3 broad categories according to its anatomical origin: disc degeneration, disc protrusions/herniations and facet degeneration. The primary role of imaging is to exclude causes of nondegenerative pain (e.g., compression fractures, tumors, neural disorders and traumatic injuries) and indicate which of the above three categories may be the primary pain generator.
This article describes procedural techniques developed through extensive experience, a thorough literature review, and feedback from spine and neurosurgeons. It is important to recognize that the techniques outlined here are among many possible options.
This paper will first review the safety profile of injection procedures as a guide to obtaining informed consent and outline the pertinent differences between lumbar and cervical injections. Then, epidural, nerve block and facet injections will each be examined separately, with a particular focus on outlining the differences between lumbar and cervical therapy.
The arsenal of interventional pain management includes many other injection procedures, such as stellate ganglion blocks and trigger point, sacroiliac joint, synovial cyst rupture, and intradiscal steroid injections. A complete review of all these procedures is beyond the scope of this article.
But by initially focusing on epidural injections/nerve blocks and facet injections—the most common procedures—the interventional musculoskeletalor neuroradiologists can subsequently expand their practices to include other procedures.
Safety profile: What you need to know for informed consent
The safety of both lumbar and cervical epidural steroid injections (ESI) and nerve blocks has been confirmed by multiple large studies ranging from 322 to 5,334 patients, with no permanent adverse procedure related outcomes and a complication rate of <1% requiring additional treatment.23–27 In all studies, both lumbar and cervical procedures were performed in outpatient settings, though not necessarily in ambulatory surgery centers. A Carm (portable or fixed) or CT was used for guidance. There is no evidence in the literature comparing fluoroscopic to CT guidance in either the lumbar or cervical spine (although many operators are passionate on their choice of imaging modality). Ultimately, operators will choose the imaging modality that best suits their technique.
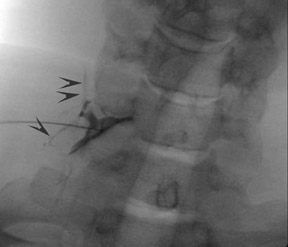
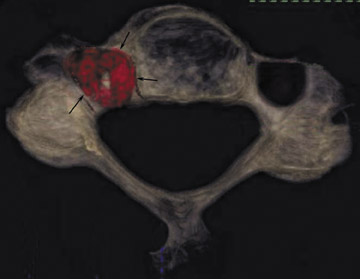
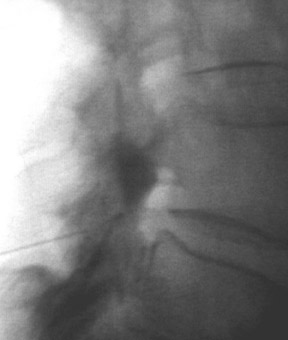
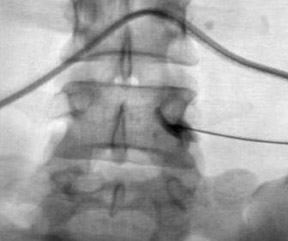
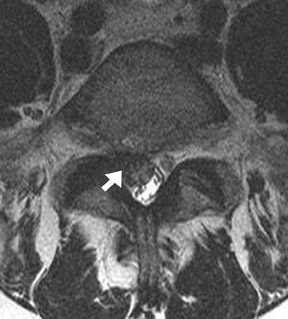
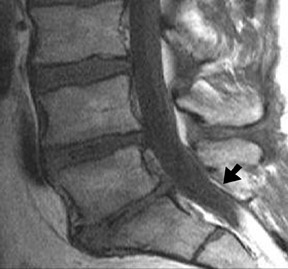
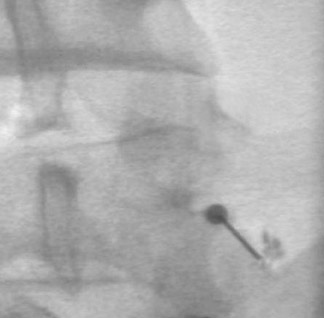
One aspect that may favor fluoroscopic over CT guidance is the risk of intravascular injections in the superior-inferior plane (Figure 1) that may not be visualized on CT. This risk is higher in the cervical spine with transforaminal injection due to: 1) tortuosity of the vertebral artery (Figure 2); 2) direct injection into the cord; and 3) injection into microvasculature that supplies the spinal cord. Isolated reports, documenting lower cord infarction and cauda equinalike symptoms,28,29 emphasize the importance of avoiding intravascular injection. As these techniques utilize contrast for guidance, complications relating to contrast administration must also be considered. In patients who are not candidates for nonionic contrast, gadolinium may be substituted.30,31
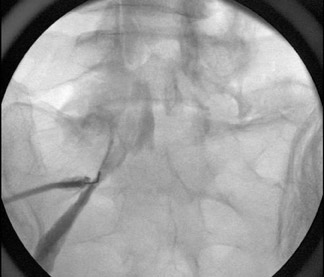
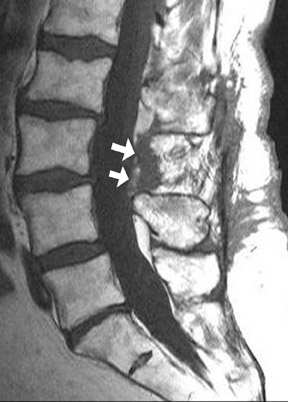
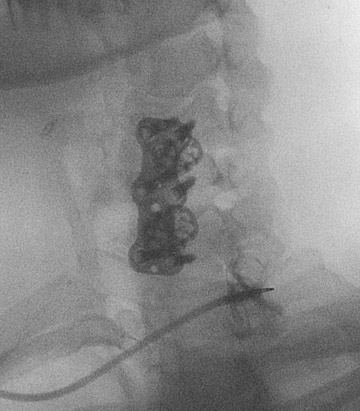
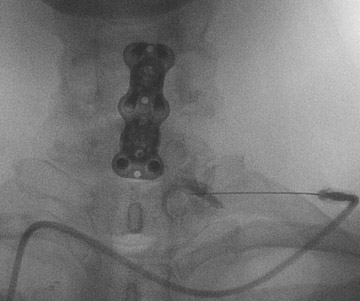
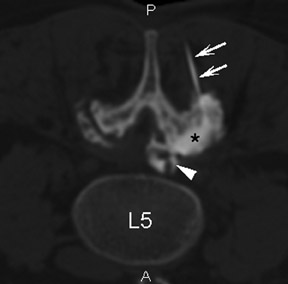
The most common complications in neck and back procedures are pain23,25 and needle misplacement, primarily in the subarachnoid space (Figure 3). Transforaminal cervical injections are more controversial. While some studies have shown their safety,32,33 many physicians have reconsidered performing these34,35 due to an unfavorable safety profile shown by other studies.36–42 Cervical nerve blocks that target the nerve along its extraspinal course using fluoroscopy may be a safer alternative.26,27
Epidural steroid injections
Rationale and patient selection
The literature is replete with articles describing the benefits of ESIs.5–22 These injections are of value to patients with both spinal stenoses and acute herniations.17,22 In cases of chronic stenosis, especially among the elderly, patients can remain functional despite a relatively high degree of pain, but they are often incapacitated by acute exacerbations. Pain management injections are very useful in combating these episodes and returning the patient to baseline function. The alternatives to injection may be surgery or, in those for whom surgery is not an option, a life of pain. The patient should understand that one injection is not a permanent cure. Back pain that returns with activity, requiring reinjection at the same location, does not mean the original injection was a failure. Many patients settle into a routine of bi- or triannual injections to control pain.
In instances of multilevel disease, selective nerve blocks may help in planning the extent of fusion surgery, or a series of successful facet blocks may shift attention to the posterior column. Surgeons may also use these procedures as a gatekeeper in selecting patients for surgery.7,43 As a tool for predicting 1-year surgical outcome, spinal injections have a reported sensitivity between 65% and 100%, a specificity between 71% and 95%, and a positive predictive value as high as 95%.7
ESIs can result in significant pain relief,18,19 decreased operative rates43 and cost savings,44 particularly in those over 65 years of age. Acute lesions and radicular pain respond better to ESI;6,8,45 disc herniations respond better than spinal stenoses (61% vs. 38%).8 In spinal stenoses, however, patient response is independent of the degree of stenosis.46 Injections may be the only effective pain relief mechanism for patients with multilevel disease (especially in the cervical spine, where there are no FDA approved devices for fusions of greater than three levels), or those who cannot undergo surgery.
Technique
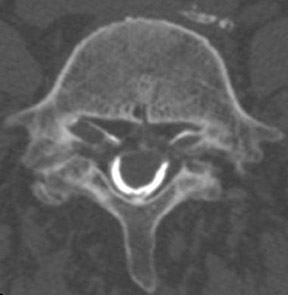
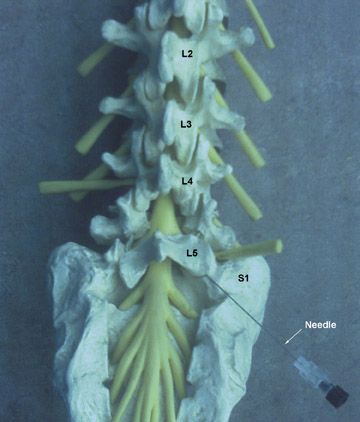
Fluoroscopic needle guidance is needed for optimum efficacy.47–49 The epidural space can be reached via caudal, interlaminar or transforaminal approaches. Of the three, the transforaminal and interlaminar approaches are employed most often (Figures 47).4,16 The caudal approach is the least used24 but easiest to perform and aimed at the sacral notch (Figure 4).
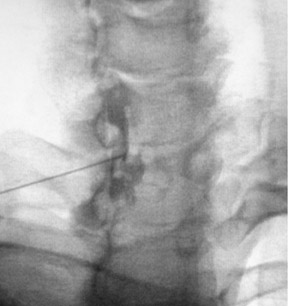
For the lumbar interlaminar approach, the patient is positioned prone on the table. Tilting the Carm at an approximately 5-degree angle ipsilateral to the side of entry and a 2-degree to 5-degree caudal/Ferguson tilt may better expose the apex of the interlaminar space (Figure 5). Optimal needle position is at the midline (Figure 5). Needle placement too far from the midline may result in the injectate remaining ipsilateral and not crossing the midline.50 In the cervical spine, due to the smaller size of the epidural space more superiorly, the injection is almost always performed at C7T1 or C6C7.42 A lateral projection usually is of little value, due to the shoulders’ obscuring the lower cervical spine. Therefore, an alternative approach entails obtaining an obliquity of approximately 45 degrees contralateral to the side of injection. Correct position is achieved when the needle tip is in the spinolaminar line (Figure 6). The injectate is then directed upward by turning the needle (with a curved tip or side hole) cephalad.
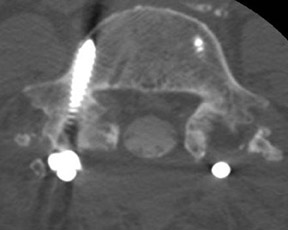
The lumbar transforaminal approach may require a steeper angulation of approximately 30 degrees ipsilateral to the entry site. A cephalad/reverse Ferguson tilt should be obtained until the endplates of the target level overlap (the angle will be most severe at L5S1 and least at L2L3). Optimal needle position is in a ‘safe triangle’ bounded by the exiting nerve at its base with the sides made up by the undersurface of the transverse process and the lateral margin of the vertebral body (Figure 7). The target point is underneath (i.e., 6 o’clock) the pedicle (Figure 7). There is a posterolateral variation to the lumbar transforaminal approach that places the needle more medially and advances the needle until it arrives at the posterior portion of the foramen on the lateral view.51 An S1 injection involves only minimal ipsilateral obliquity and a 20-degrees-of-cephalad tilt of the Carm until the posterior and anterior S1 foramina overlap. A lateral projection is used to ensure that the needle is not advanced too deeply beyond the sacrum.
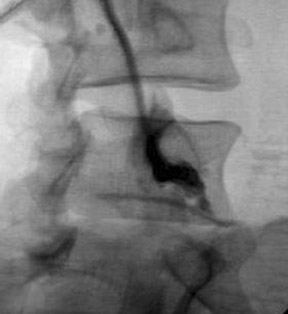
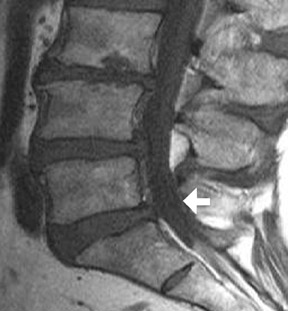
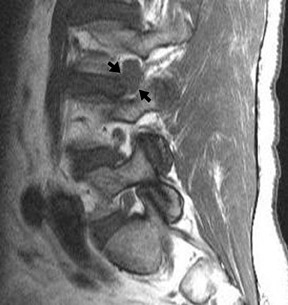
Approach selection depends on the location of disc pathology and the nerves involved. A patient with subarticular or posterolateral impingement on a nerve root (Figure 8) would benefit from a transforaminal injection at that level or the level below (as flow in the epidural space is often preferentially cephalad, along the nerve roots as they track back to the thecal sac). A patient with a broad-based disc bulge may benefit more from an interlaminar injection, although the location of the injection must consider that the epidural space may be located either above or below the disc (Figure 9). In the postoperative spine where laminectomy has removed the posterior epidural space, transforaminal injections or an approach above or below the surgical level may be necessary (Figure 10). Foraminal herniations present a particular challenge. While they may be easily accessed by the transforaminal route, the narrowing of the foramen may make such injections very painful (Figure 11). Systematic reviews of foraminal injections as well as our own experience have found marginal benefit over interlaminar injections in pain relief.4,16
Needles for ESI
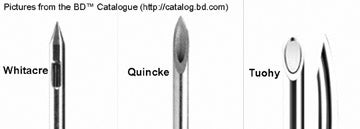
The type and size of needles available for epidural injections vary considerably; there is little evidence to support choosing one needle over another (Figure 12). There are curved and straight needles. Among the straight variety, a 22-gauge spinal Quincke (end hole/ spinal) needle may be used in the lumbar spine, while a 25-gauge needle may be used in the cervical spine. Many operators prefer a 22-gauge Whitacre or Pajunk (sidehole) needle in the cervical spine; some use this needle in the lumbar spine, as well. A blunt-tip Whitacre or Pajunk needle will require an introducer to penetrate the skin. A 1.5-inch, 18-gauge standard injection needle is adequate for this purpose, although some manufacturers make special introducer sheath/needle combinations. The curved (Tuohy) needle is an alternative to the straight needle. The use of a curved needle requires a minimum of 22-gauge. Ultimately, the choice of needles depends on operator comfort.
Medication for ESI
The choice of injectate is operator dependent, with many combinations reported. The injectate consists of a contrast agent followed by local anesthesia and a steroid. Some operators inject the local anesthesia separately, followed by the steroid, while others mix the two.
Contrast is used to verify epidural location and to indicate the distribution of injectate. Some physicians use contrast as a volume expander while others prefer saline for this use. The contrast is typically nonionic and lowosmolar. In patients with contrast allergies gadolinium can be safely used in most lumbar procedures.30,31,52,53 If using gadolinium, the amount should be just enough to document epidural injection. Gadolinium should not be used as a substitute for volume expander. The typical amount of contrast or contrastsaline mixture used for either cervical or lumbar interlaminar epidurography is 4 cc to 5 cc (less in nerve blocks; see below). A smaller amount will not provide sufficient contrast for an epidurogram to evaluate for adhesions or distribution of injectate. For coding purposes, an epidurogram is considered to have been performed when approximately 4 cc to 5 cc of contrast is injected regardless of the route (transforaminal or interlaminar). The report, CPT code, and amount billed must be adjusted if an epidurogram is not performed. The amount of contrast injected may be reduced in spinal stenosis. Many patients will feel pressure or leg cramping from almost any volume, no matter how small. Patients undergoing first-time injections may confuse this with pain. Careful questioning and reassurance that pressure is normal will be adequate in most cases. The injectate volume should be reduced if significant pain is experienced.
The choice and amount of epidural anesthetic agent is subjective. Lidocaine 1% and 2%; and Marcaine 0.25%, 0.5% and 1% have all been used. The epidural anesthetic agent should not contain epinephrine. Marcaine will have a stronger effect than lidocaine. While lidocaine is FDA-approved for use in the epidural and intrathecal space, Marcaine is used off label for this purpose. Generally, the stronger the local anesthesia, the greater the chance the patient will have difficulty ambulating and may fall after a procedure. The use of Marcaine is generally less prevalent in the literature6,23,25,30,43,46 and there have been no studies on the use of Marcaine in ESIs. Regardless of the type of epidural anesthetic agent, all patients receiving such an agent can not drive themselves immediately after the procedure to avoid the potential of foot drop while driving. Patients who do not have an accompanying driver should be kept in the office for 60 minutes to 120 minutes.
In the author’s practice 3 cc to 4 cc of lidocaine 1% is used. In cases where injectate volume must be reduced (due to spinal stenosis) but a full anesthetic effect is still desired, 2 cc of lidocaine 2% may be an alternative (injectate amounts for nerve block are reduced). Evaluating the patient’s response to the anesthetic agent while the patient is still monitored in the suite may indicate whether the injection correctly targeted the pain generator. Many operators do not use epidural anesthetic agents in the cervical spine due to the risk of anesthesia in the C3, C4 and C5 regions of the cord responsible for diaphragmatic function. These areas, if anesthetized, may cause diaphragmatic paralysis and respiratory arrest.
Of the several steroid preparations available, Dexamethasone sodium phosphate (Decadron®, Sicor Pharmaceuticals Inc, Irvine, CA) is the only steroid FDA-approved for intravenous use. In theory, it would be the safest in case of accidental intravenous administration. However, due to the short-acting nature of dexamethasone, it has not gained widespread use in pain management as evidenced by the fact that there have been very few reports on its effects as compared with other preparations.54 Of the longeracting preparations, the most soluble and least particulate is Betamethasone sodium phosphate (Celestone Soluspan®, Schering, Kenilworth, NJ). 6 mg of Betamethasone is equivalent to 0.75 of Dexamethasone. It will not elicit the arachnoiditis that may occur with other steroids in the event of accidental intrathecal injection.55,56 The greater solubility of Celestone compared to other preparations results in a lower chance of embolism in the event of accidental intravascular administration.57 Typically, 12 mg of Celestone is used (less in nerve blocks; see below). In vitro data also show that more soluble steroids (such as Decadron and Celestone) are safer than particulate steroids (DepoMedrol® and Kenalog®) in the cervical spine.58
Other steroid preparations are methylprednisolone acetate (DepoMedrol, Pfizer, New York, NY) and triamcinolone acetonide (Kenalog, BristolMyers Squibb, Princeton, NJ). A typical dose is 80 mg for both (less in nerve blocks; see below) which is equivalent to 12 mg of betamethasone. DepoMedrol and Kenalog are semi-particulate solutions that are less soluble than Celestone and, therefore, theoretically may have poorer spread in the epidural space. DepoMedrol is available as an 80mg preparation that may be helpful if the injectate volume must be reduced due to spinal stenosis. There have been reports of arachnoiditis due to the neurotoxic polyethylene glycol component of methylprednisolone acetate.56,58,60 There has been a report of Kenalog crystals found in the epidural space at surgery in patients with recent ESIs.61
The literature is conflicting as to whether Kenalog or Celestone is better for pain relief,62,63 with some suggesting that the crystallization of Kenalog provides better longterm benefit62 and others noting that the soluble properties of Celestone provide quicker onset of action.63 It is important for both the operator and patient to understand that the steroid may take up to 3 days to begin providing symptomatic relief.
Nerve block injections
Rationale and patient selection
Nerve blocks may be performed as part of the workup for potential surgery as both diagnostic and therapeutic procedures. There is no agreement in the literature on what defines a positive nerve block result or “good” pain relief. Smaller volumes are used in nerve blocks to limit the effect to only one nerve. Patients may have a single or multiple level nerve block. Multiple blocks may be performed at the same session or sequentially (but still on the same day) until the pain generator is found. When these are performed sequentially, the patient is brought into the procedure suite for the first block. Thereafter they are monitored in an observation area for approximately 30 minutes where their response to the anesthetic agent is assessed. If the patient experiences less than approximately 80% relief, then the next nerve level is treated (on the same day), and so on. Once approximately 80% relief is obtained, no further injections are needed as the patient will not be able to distinguish further relief. If a total of approximately 80% pain relief is not attained, another source of pain should be considered. Bilateral blocks can be performed, but care should be taken as the patient may have difficulty ambulating after these injections. For this reason some operators do not perform bilateral nerve blocks on the same day.
Nerve block technique, medication, and needles
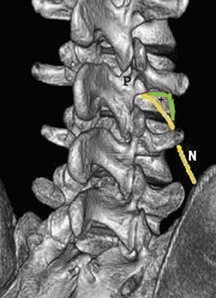
Injectate consists of 1 cc of a contrast agent, a half-dose of steroid compared to the ESIs above and 1 cc of lidocaine 2%. Broadly speaking, the technique for lumbar nerve root injection is similar to the transforaminal injection. Some variation exists in the lumbar approach; some operators may attempt to position the needle tip more laterally compared with a transforaminal ESIs to prevent reflux into the epidural space. Cervical extraforaminal nerve root blocks may be performed safely with correct technique:26,27 The Carm is tilted approximately 20 degrees to 30 degrees ipsilateral to the side of choice, with the target point at the junction of the middle and lateral third of the articular pillar just below the foramen of the nerve root to be treated (Figure 13).
Facet injections
Rationale and patient selection
Facet pain often overlaps disc pain and may coexist with disc disease. Facets are synovial joints and, therefore, they can be affected by arthritis in a fashion similar to other synovial joints (e.g., the knee, hip, etc.). Symptoms include pain in the paraspinal region, buttocks, thigh or shoulder in cervical spine; pain worsened on hyperextension following flexion and pain accompanied by paraspinal tenderness. Age, a prior history of low back pain, abnormal gait, absence of leg pain, muscle spasms, and aggravation of pain on valsalva may indicate pain with its origin in the facet joint.63
Medial branch block injections are often directed at groups of facets. There is a known placebo effect with single blocks64–66 and therefore, prior to surgery or rhizotomy a patient should have two successful (80% relief) blocks.64–66 The decision on whether to perform an intraarticular injection or medial branch facet nerve block depends on several factors: Medial branch blocks are more useful in patients who are candidates for rhizotomy, where uncertainty exists over the origin of back pain and in those where the facets are so overgrown and deformed by osteophytes that intraarticular injection is not possible.67–70 In the cervical spine, intraarticular injections have shown little efficacy whereas medial branch blocks have shown benefits.14
Technique
Radiologic guidance (fluoroscopy or CT) is essential, as facets at different levels have different orientations (Figures 14, 15). As little as 1 cc of injectate can rupture a nondiseased facet capsule.71 Studies have postulated that facet capsule rupture is associated with increased pain relief possibly due to injectate bathing the medial ramus feeding the facet and spreading to the epidural space.67,68,71 Despite the presumed benefits of extracapsular spread of injectate, initial needle placement within the facet joint provides better pain relief than extracapsular injection.68 There is a dual innervation to each lumbar facet joint. Each facet is innervated by the medial branches of the posterior rami of the spinal nerves above and below the joint. Thus, the L4L5 facet is innervated by the L5 medial branch (coursing over the L5 transverse process) and the L4 medial branch (coursing over the L4 transverse process). An exception to this rule is the C2C3 facet joint, which is innervated by the third occipital nerve and the C1 medial branch that courses superolaterally to the C1 foramen. The medial branch of levels other than C2C3 and S1 is approached by tilting the Carm at approximately 20 degrees ipsilateral to the nerve in question (Figure 16) and aiming for the junction of the transverse process and the superior articulating process (i.e., the ear and nose of the “Scottydog”). Medications and needles for medial branch blocks are similar to those described for nerve blocks. In all cases contrast (±0.5cc) should be injected to exclude a vascular injection and, in intraarticular injections, to perform a facet arthrogram (if this is not performed, the report and CPT charge should be adjusted). This is then followed with a 1:1 steroid/local anesthetic mix at half the volume of a nerve block (i.e., the total steroid/steroid/local anesthetic volume should be approximately 1 cc). Many operators will use 20 mg Depo-Medrol per facet joint injection as Depo-Medrol is FDA-approved for joint injections and there is little risk of intrathecal injection. For similar reasons, either Marcaine or lidocaine may be used in facet injections. Some operators only use medial branch blocks as a diagnostic technique, in which case no steroid is injected.
Conclusion
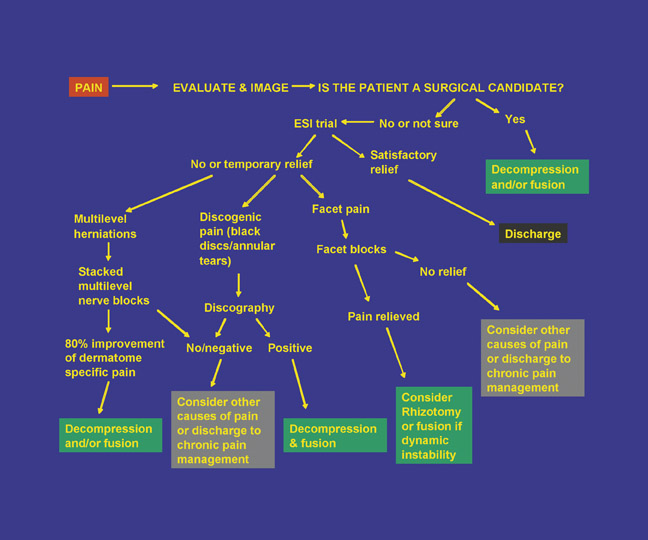
Basic pain management procedures benefit patients who are both operative and non-operative candidates as determined by clinical parameters and imaging (Figure 17). In those patients who are not operative candidates, spinal injections can help identify anterior column (disc herniations and degeneration) versus posterior column (facet) disease. Minimally invasive techniques can produce long-term relief. In patients who are operative candidates, pain-relieving injections can assist in identifying the level of surgery in multilevel disease and temporizing pain while the patient awaits surgical scheduling.
REFERENCES
- Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007: 15;32(16):1754-1760.
- Manchikanti L, Boswell MV, Singh V, et al. Comprehensive evidencebased guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009;12:699-802.
- Manchikanti L, Boswell MV, Datta S, et al. Comprehensive Review of Therapeutic Interventions in Managing Chronic Spinal Pain. Pain Physician. 2009; 12(4):E123-198.
- Parr AT, Diwan S, Abdi S. Lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain: a systematic review. Pain Physician. 2009;12:163-188.
- Gilbert FJ, Grant AM, Gillan MG, et al. Low Back Pain: Influence of Early MR Imaging or CT on Treatment and Outcome Multicenter Randomized Trial. Radiology. 2004; 231:34–351.
- Ridley MG, Kingsley GH, Gibson T, Grahame R. Outpatient lumbar epidural corticosteroid injection in the management of sciatica. Br J Rheumatol. 1988; 27:295-299.
- Young IA, Hyman GS, PackiaRaj LN, Cole AJ. The use of lumbar epidural/transforaminal steroids for managing spinal disease. J Am Acad Orthop Surg. 2007;15:228-238.
- Berman AT, Garbarino JL Jr, Fisher SM, Bosacco SJ. The effects of epidural injection of local anesthetics and corticosteroids on patients with lumbosciatic pain. Clin Orthop Relat Res. 1984; 144-151.
- Cyteval C, Fescquet N, Thomas E, et al. Predictive factors of efficacy of periradicular corticosteroid injections for lumbar radiculopathy. AJNR Am J Neuroradiol. 2006;27:978-982.
- Holve RL. Oral steroids in initial treatment of acute sciatica. J Am Board Fam Med. 2008; 21:469-474.
- Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. 2007:1;32(19):2127-2132.
- Franklin GM, Stover BD, Turner JA, et al. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine. 2008:15;33:199-204.
- Abdi S. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician. 2007;10:185-212.
- Falco FJ, Erhart S, Wargo BW, et al. Systematic review of diagnostic utility and therapeutic effectiveness of cervical facet joint interventions. Pain Physician. 2009;12:323-344.
- Benyamin RM, Singh V, Parr AT, et al. Systematic review of the effectiveness of cervical epidurals in the management of chronic neck pain. Pain Physician. 2009;12:137-157.
- Buenaventura RM, Datta S, Abdi S, Smith HS. Systematic review of therapeutic lumbar transforaminal epidural steroid injections. Pain Physician. 2009; 12:233-251.
- Rivest C, Katz JN, Ferrante FM, Jamison RN. Effects of epidural steroid injection on pain due to lumbar spinal stenosis or herniated disks: a prospective study. Arthritis Care Res. 1998;11:291-297.
- Lutz GE, Vad VB, Wisneski RJ. et al. Fluoroscopic transforaminal lumbar epidural steroids: an outcome study. Arch Phys Med Rehabil. 1998;79:1362-1366.
- Vad VB, Bhat AL, Lutz GE, et al. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine. 2002:1; 27:11-16.
- Viton JM, Rubino T, PerettiViton P, et al. Shortterm evaluation of periradicular corticosteroid injections in the treatment of lumbar radiculopathy associated with disc disease. Rev Rhum Engl Ed. 1998;65:195-200.
- Karppinen J, Malmivaara A, Kurunlahti M, et al. Periradicular infiltration for sciatica: a randomized controlled trial. Spine. 2001:1;26:1059-1067.
- Botwin KP, Gruber RD, Bouchlas CG, et al. Fluoroscopically guided lumbar transformational epidural steroid injections in degenerative lumbar stenosis: an outcome study. Am J Phys Med Rehabil. 2002;81: 898-905.
- Waldman SD. Complications of cervical epidural nerve blocks with steroids: a prospective study of 790 consecutive blocks. Reg Anesth. 1989; 14:149-151.
- Johnson BA, Schellhas KP, Pollei SR. Epidurography and therapeutic epidural injections: technical considerations and experience with 5334 cases. AJNR Am J Neuroradiol. 1999;20:697-705.
- Botwin KP, Gruber RD, Bouchias CG, TorresRamos FM, Freeman IL, Slaten WK. Complications of fluoroscopically guided transforaminal lumbar epidural injections. Arch Phys Med Rehabil. 2000;81:1045-1050.
- Schellhas KP, Pollei SR, Johnson BA, et al. Selective cervical nerve root blockade: experience with a safe and reliable technique using an anterolateral approach for needle placement. AJNR Am J Neuroradiol. 2007;28:1909-1914.
- Pobiel RS, Schellhas KP, Eklund JA, et al. Selective cervical nerve root blockade: prospective study of immediate and longer term complications. AJNRAmJ Neuroradiol. 2009;30:507-511.
- Somayaji HS, Saifuddin A, Casey AT. Spinal cord infarction following therapeutic computed tomographyguided left L2 nerve root injection. Spine. 2005 Feb 15;30:E106-108.
- Hettsa SW, Narvida J, Singha T. et al. Association between Lumbar Epidural Injection and Development of Acute Paraparesis in Patients with Spinal Dural Arteriovenous Fistulas.AJNR Am J Neuroradiol. 2007;28:581-583.
- Safriel YI, Ali M, Hayt M and Ang R. Gadolinium use in spine procedures for patient with allergy to iodinated contrast – experience of 127 procedures. AJNR Am J Neuroradiol. 2006 JunJul;27:1194-1197.
- Shetty SK, Nelson EN, Lawrimore TM, Palmer WE. Use of gadolinium chelate to confirm epidural needle placement in patients with an iodinated contrast reaction. Skeletal Radiol. 2007;36:301-307.
- Ma D, Gilula LA, Riew KD. Complications of fluoroscopically guided extraforaminal cervical nerve blocks. An analysis of 1036 injections. J Bone Joint SurgAm. 2005; 87(5):1025-1030.
- Gilula LA, Ma D. A cervical nerve block approach to improve safety. AJR Am J Roentgenol. 2007;189;563-565.
- Raghavendra M, Patel V. Should we cease performing transforaminal injections? Reg Anesth Pain Med. 2005 MarApr;30(2):207-208.
- Scanlon GC, MoellerBertram T, Romanowsky SM, Wallace MS. Cervical transforaminal epidural steroid injections: more dangerous than we think? Spine. 2007:15;32:1249-1256.
- Wallace MA, Fukui MB, Williams RL, et al. Complications of cervical selective nerve root blocks performed with fluoroscopic guidance. AJR Am J Roentgenol. 2007;188:1218–1221.
- Hodges SD, Castleberg RL, Miller T, Ward R, Thornburg C. Cervical epidural steroid injection with intrinsic spinal cord damage. Two case reports. Spine. 1998; 1;23:2137-2142.
- Ludwig MA, Burns SP. Spinal cord infarction following cervical transforaminal epidural injection: a case report. Spine. 2005:15;30:E266-268.
- Beckman WA, Mendez RJ, Paine GF, Mazzilli MA. Cerebellar herniation after cervical transforaminal epidural injection. Reg Anesth Pain Med. 2006;31: 282-285.
- Tiso RL, Cutler T, Catania JA, Whalen K. Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine J. 2004; 4:468-474.
- Huntoon MA. Anatomy of the cervical intervertebral foramina: vulnerable arteries and ischemic neurologic injuries after transforaminal epidural injections. Pain. 2005; 117:104-111.
- Abbasi A, Malhotra G, Malanga G, Elovic EP, Kahn S. Complications of interlaminar cervical epidural steroid injections: a review of the literature. Spine. 2007;1;32:2144-2151.
- Riew KD, Yin Y, Gilula L, et al. The effect of nerveroot injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, doubleblind study.J Bone Joint Surg Am. 2000;82A:1589-1593.
- Karppinen J, Ohinmaa A, Malmivaara A, et al. Cost effectiveness of periradicular infiltration for sciatica: subgroup analysis of a randomized controlled trial. Spine. 2001:1;26:2587-2595.
- HeyseMoore GH. A rational approach to the use of epidural medication in the treatment of sciatic pain. Acta Orthop Scand. 1978;49:366-370.
- Campbell MJ, Carreon LY, Glassman SD, McGinnis MD, Elmlinger BS. Correlation of spinal canal dimensions to efficacy of epidural steroid injection in spinal stenosis. J Spinal Disord Tech. 2007;20:168-171.
- White AH, Derby R, Wynne G. et al. Epidural injections for the diagnosis and treatment of lowback pain. Spine. 1980;5:78-86.
- Renfrew DL, Moore TE, Kathol MH, et al. Correct placement of epidural steroid injections: fluoroscopic guidance and contrast administration. AJNR Am J Neuroradiol. 1991;12:1003-1007.
- Bartynski WS, Grahovac SZ, Rothfus WE. et al. Incorrect needle position during lumbar epidural steroid administration: inaccuracy of loss of air pressure resistance and requirement of fluoroscopy and epidurography during needle insertion. AJNR Am J Neuroradiol. 2005;26:502-505.
- Whitlock EL, Bridwell KH, Gilula LA. Influence of needle tip position on injectate spread in 406 interlaminar lumbar epidural steroid injections. Radiology. 2007; 243:804-811.
- Lee IS, Kim SH, Lee JW, et al. Comparison of the temporary diagnostic relief of transforaminal epidural steroid injection approaches: conventional versus posterolateral technique.AJNR Am J Neuroradiol. 2007;28:204-208.
- Skalpe IO. Is it dangerous to inject magnetic resonance contrast media into the subarachnoid space? Eur Radiol. 1998;8:427.
- Tali ET, Ercan N, Krumina G, et al. Intrathecal gadolinium (gadopentetate dimeglumine) enhanced magnetic resonance myelography and cisternography: results of a multicenter study.Invest Radiol. 2002;37:152-159.
- Dreyfuss P, Baker R, Bogduk N. Comparative effectiveness of cervical transforaminal injections with particulate and nonparticulate corticosteroid preparations for cervical radicular pain.Pain Med. 2006; 7:237-242.
- Nelson DA. Intraspinal therapy using methylprednisolone acetate: twentythree years of clinical controversy. Spine. 1993;18:278–286.
- Abram SE, O’Connor TC. Complications associated with epidural steroid injections. Reg Anesth. 1996;21:149–162.
- Okubadejo G, Talcott M, Schmidt R, et al. Perils of intravascular methylprednisolone injection into the vertebral artery: an animal study. J Bone Joint Surg Am. 2008;90:1932–1938.
- Silbergleit R, Bharat MA, William SP, et al. Imagingguided injection techniques with fluoroscopy and CT for spinal pain management. Radiographics 2001; 21:927–939.
- Nelson DA. Dangers from methylprednisolone acetate therapy by intraspinal injection. Arch Neurol. 1988; 45:804–806.
- Botwin KP, Castellanos R, Rao S, et al. Complications of fluoroscopically guided interlaminar cervical epidural injections. Arch Phys Med Rehabil. 2003; 84:627–633.
- Stanczak J, Blankenbaker DG, De Smet AA, Fine J. Efficacy of epidural injections of Kenalog and Celestone in the treatment of lower back pain. AJR Am J Roentgenol. 2003; 181:1255–1258.
- Blankenbaker DG, De Smet AA, Stanczak JD, et al. Lumbar radiculopathy: treatment with selective lumbar nerve blocks—comparison of effectiveness of triamcinolone and betamethasone injectable suspensions. Radiology. 2005;237:738741.
- Schwarzer AC, Aprill CN, Derby R, et al. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine. 1994:15;19:1132-1137.
- Jackson RP, Jacobs RR, Montesano PX. 1988 Volvo award in clinical sciences. Facet joint injection in lowback pain. A prospective statistical study. Spine. 1988;13:966-971.
- Stanley D, McLaren MI, Euinton HA, Getty CJ. A prospective study of nerve root infiltration in the diagnosis of sciatica. A comparison with radiculography, computed tomography, and operative findings. Spine. 1990 Jun;15:540-543.
- Schwarzer AC, Aprill CN, Derby R, et al. The falsepositive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain. 1994;58: 195-200.
- Dory MA. Arthrography of the lumbar facet joints. Radiology. 1981;140:23-27.
- Raymond J and Dumas JM. Intraarticular facet block: diagnostic test or therapeutic procedure? Radiology. 1984;151:333-336.
- Moran R, O’Connell D, Walsh MG. The diagnostic value of facet joint injections. Spine. 1988;13:1407-1410.
- Kaplan M, Dreyfuss P, Halbrook B, et al. The ability of lumbar medial branch blocks to anesthetize the zygapophysial joint. A physiologic challenge. Spine. 1998:23:1847-1852.
- Lynch MC and Taylor JF. Facet joint injection for low back pain. A clinical study. J Bone Joint Surg Br. 1986;68:138-141.
Related Articles
Citation
. Lumbar and cervical pain management procedures: When and how to do them. Appl Radiol.
December 17, 2010