Not All Prostate-Specific Membrane Antigen Imaging Agents Are Created Equal: Diagnostic Accuracy of Ga-68 PSMA-11 PET/CT for Initial and Recurrent Prostate Cancer
By Purysko AS, Abreu AL, Lin DW, Punnen S

This is an open access article under the terms of the http://creativecommons.org/licenses/by/4.0/ License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited. This review article is sponsored by Telix Pharmaceuticals.
Abstract
Positron emission tomography (PET) radiotracers that target prostate-specific membrane antigen (PSMA), a transmembrane protein overexpressed in prostate cancer (PCa) cells, are highly sensitive and specific for the detection of metastatic PCa. The radioactive PET imaging agent Ga-68 PSMA-11 has demonstrated higher PCa detection rates compared with conventional imaging techniques, leading to its increased use in the diagnosis of PCa. In this review of literature published between February 2015 and December 2022, of 76 studies in >5000 men with PCa, we examined the accuracy and clinical use of Ga-68 PSMA-11 PET for the initial staging of PCa, assessment of biochemical recurrence (BCR), and how this technique may affect the clinical management of PCa. The majority of studies evaluating Ga-68 PSMA-11 PET for primary staging and for BCR demonstrated a sensitivity ≥80% and a specificity ≥90%. Ga-68 PSMA-11 PET led to a change in clinical management in 19% to 52% and 16% to 75% of patients with primary PCa and BCR, respectively. Variations in diagnostic accuracy parameters were observed among studies but were anticipated given differences in patient characteristics (eg, PSA, lesion sizes) and study designs. No serious adverse events were noted with Ga-68 PSMA-11 PET. Overall, Ga-68 PSMA-11 offers high sensitivity, is well tolerated, and can result in clinical management changes for patients with primary PCa and BCR.
Introduction
Prostate cancer (PCa) is diagnosed in approximately 1.3 million men each year and represents the second most common cancer in men worldwide.1 The American Cancer Society estimated 288,300 new cases and 34,700 deaths from PCa in the United States in 2023.2 The 5-year survival rate is 99% for patients with localized/regional PCa, but only 32% for patients with distant metastasis.3
PCa recurrence, defined as an increase in prostate-specific antigen (PSA) levels after treatment, occurs in up to 90% of cases, depending on initial risk categorization and definitive therapy. Biochemical recurrence (BCR) is defined as a PSA level of 0.2 ng/mL followed by a confirmatory PSA level of ≥0.2 ng/mL after radical prostatectomy (RP) and nadir PSA level + 2.0 ng/mL after radiotherapy.4 Patients with preoperative PSA levels of <2.6 ng/mL, 2.6 to 10 ng/mL, and >10 ng/mL are expected to have recurrence rates of 10%, 20%, and 50%, respectively.5 Approximately 40% to 90% of patients with high-risk features develop BCR following prostatectomy3,6 and 30% to 50% experience BCR following radiation therapy.7 However, multinomial nomograms based on other clinical factors, such as Gleason grade group and clinical stage, provide more accurate estimates of BCR.8
PCa is diagnosed via biopsy of the prostate, with imaging playing an important role in its diagnosis and management. However, staging for higher-risk disease is often performed using conventional imaging with computed tomography (CT) and bone scintigraphy, which have suboptimal sensitivities for detecting metastases for initial staging.9 CT and Tc-99m methyl diphosphonate bone scintigraphy are routinely used to stage disease in patients with confirmed PCa and to assess suspected PCa recurrence. These methods, however, have limited sensitivity in detecting metastatic disease, particularly when patients have smaller lesions and lower PSA levels.10
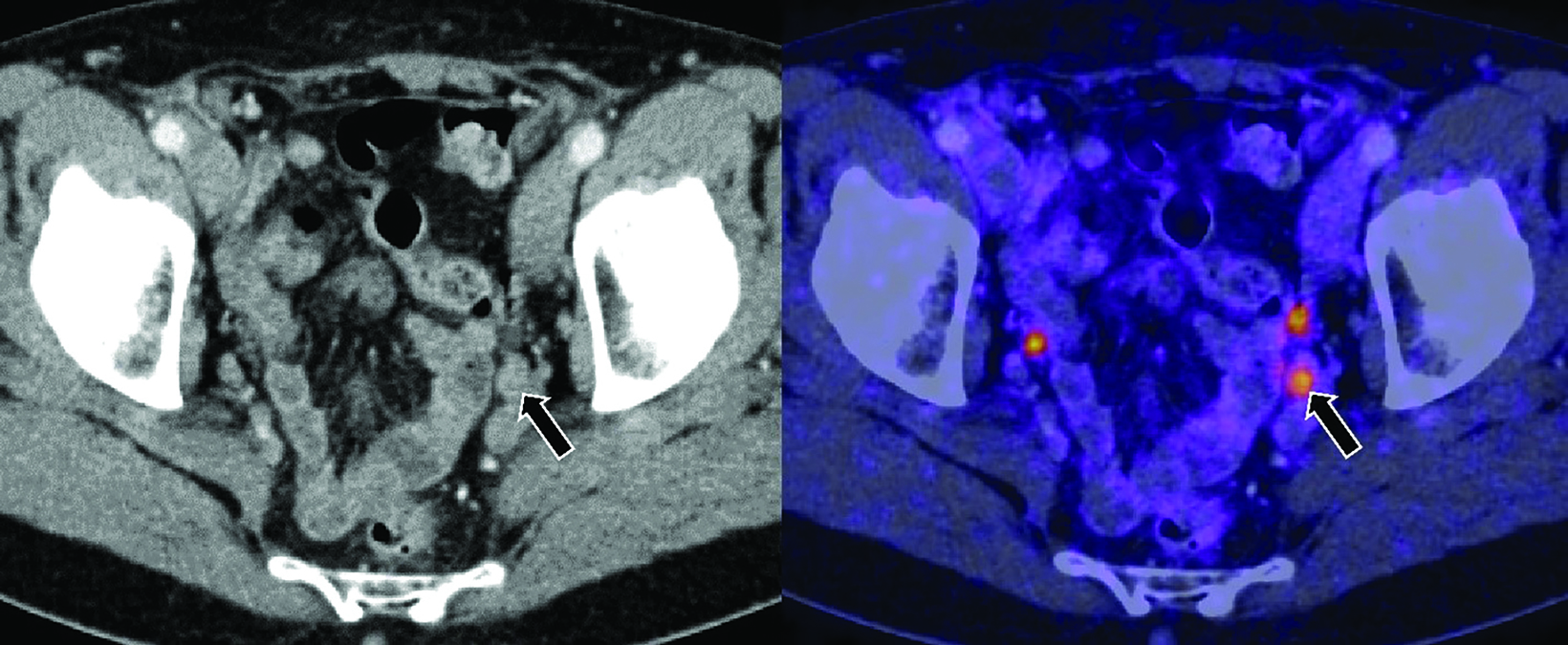
Recent advances in diagnostic imaging have overcome these limitations. Positron emission tomography (PET) radiotracers that target prostate-specific membrane antigen (PSMA), a transmembrane protein overexpressed in PCa cells, are highly sensitive and specific, with a high detection rate for metastatic PCa lesions. Thus, PSMA radiotracers are recommended for PET imaging of PCa without the prerequisite use of conventional imaging.11 Ga-68 PSMA-11 is one such radioactive PET imaging agent that has demonstrated higher PCa detection rates compared with conventional imaging techniques, leading to its increased use along with other PET imaging agents.12 Figure 1 shows an example of a pelvic node in the setting of BCR that was positive on Ga-68 PSMA-11 PET/CT, but negative on CT.
Ga-68, a β+ emitting radionuclide, is one of the most common radioisotopes used in PET scans worldwide.13 PET imaging using Ga-68 PSMA-1114 was approved by the US Food and Drug Administration (FDA) as the first PSMA-targeted imaging agent on December 1, 2020, followed by approval of a kit for the preparation of Ga-68 PSMA-11 (TLX591-CDx) on December 17, 2021 for widespread commercial use.15
Ga-68 PSMA-11 is approved for the detection of suspected metastasis in the initial staging of patients with PCa and the identification of suspected PCa BCR after treatment.14 Ga-68 PSMA-11 is also approved in the US to identify and select patients who are candidates for FDA-approved PSMA-directed radioligand therapy. A recent study also reported a significant effect of Ga-68 PSMA-11 on the staging and management of PCa across all relevant clinical scenarios, including patients with PSA below the threshold for BCR, those with known metastatic or advanced castration-resistant disease, and those who have undergone primary treatments other than surgery or radiotherapy.16
Herein we review the available literature to assess the sensitivity and specificity of Ga-68 PSMA-11 PET for PCa imaging along with its safety and clinical use for PCa management. Literature searches were conducted using PubMed to identify published studies relevant to the use of Ga-68 PSMA-11 PET for the detection and staging of primary PCa and to detect and localize BCR. Search terms included “primary prostate cancer,” “prostate cancer,” “PSMA,” “PET,” “staging,” and “biochemically recurrent.” The initial search was limited to studies published in English from February 2015 to November 2021. A second search was conducted to identify articles from November 2021 to December 2022 using the same search terms. Overall, 75 studies in >5000 men with PCa were identified that examined the accuracy and clinical use of Ga-68 PSMA-11 PET for the initial staging of PCa and assessing BCR (Figure 2).
Primary staging of PCa using Ga-68 PSMA-11
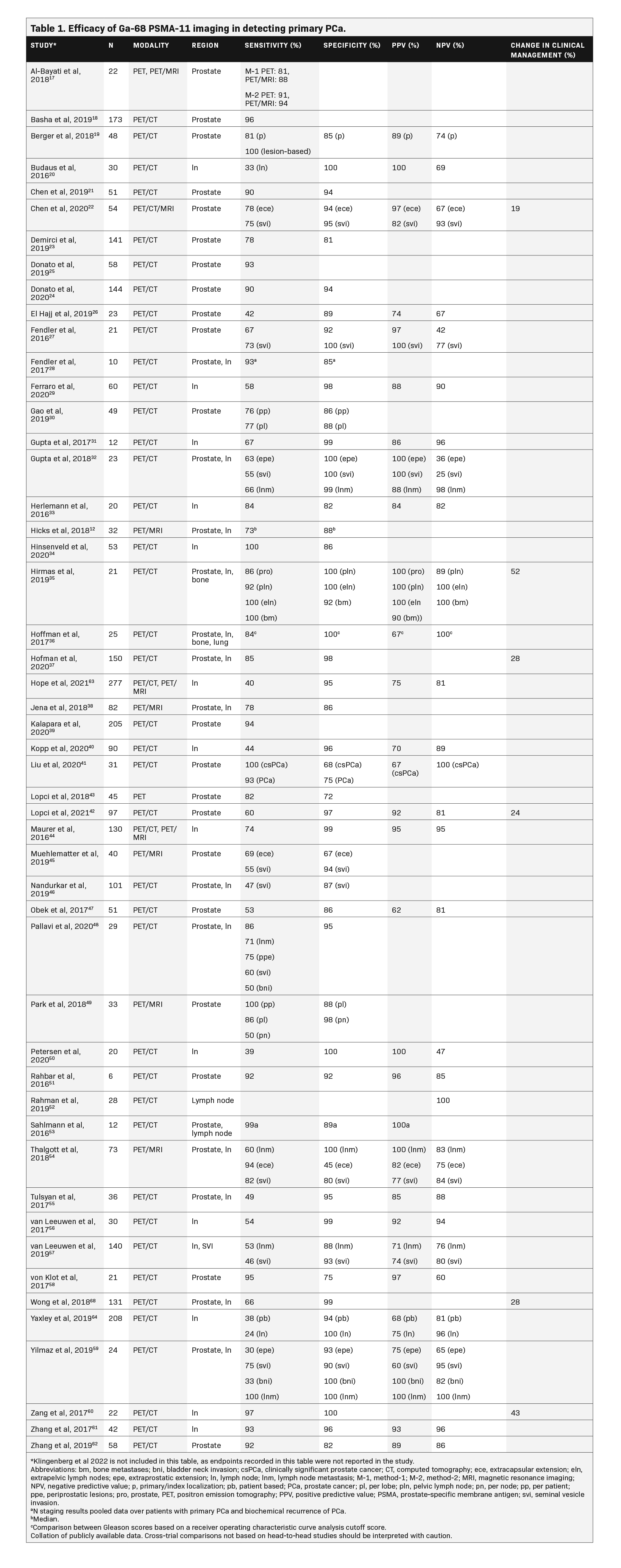
Table 1 summarizes the results of studies that assessed Ga-68 PSMA-11 for the primary staging of PCa. The majority of studies demonstrated a sensitivity ≥80% and a specificity ≥90%. Sensitivity of Ga-68 PSMA-11 ranged from 30% to 100% for detecting PCa, 24% to 100% for detecting cancer in the lymph nodes, and 84% to 100% for detecting bone metastases; its specificity ranged from 45% to 100% for the prostate, 82% to 100% for the lymph nodes, and 92% to 100% for bone metastases. The positive predictive value (PPV) ranged from 60% to 100%, and the negative predictive value (NPV) ranged from 25% to 100% in both localized and metastatic PCa, with the majority of these studies reporting values in the upper range.12,17-64Additionally, a prospective study highlighted the prognostic value of 68-Ga PSMA-11 PET/CT versus conventional imaging with 99mTc bone scintigraphy and CT for primary staging in 247 high-risk patients with PCa treated with RP. Primary staging with 68-Ga PSMA-11 PET/CT resulted in a significantly lower biochemical recurrence risk after RP vs conventional imaging, likely due to improved selection of patients for RP.65
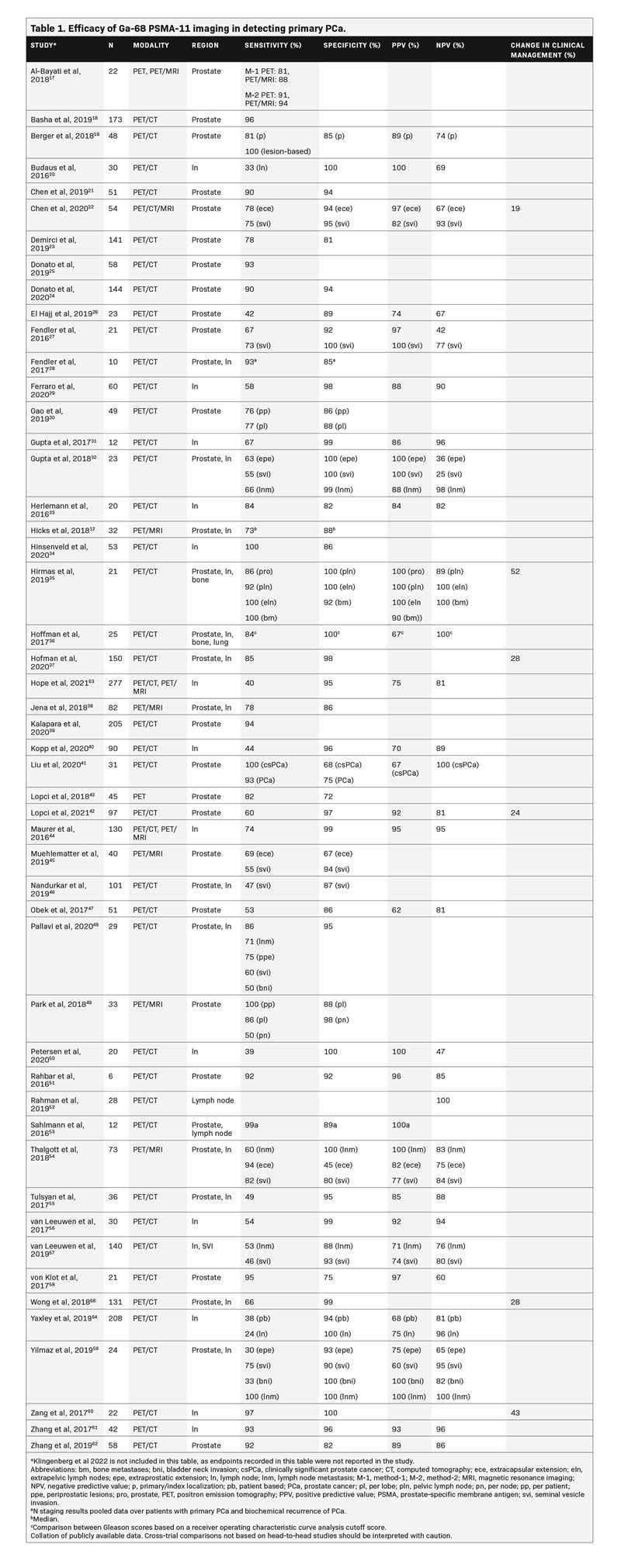
Initial PCa detection and T staging
In a study evaluating 144 patients (median PSA 8.6 ng/mL), Ga-68 PSMA-11 PET/CT was compared with multiparametric MRI (mpMRI) for the detection of localized PCa, with biopsy histopathology used as a reference standard for the full cohort and RP specimen used as the reference standard in a subset of patients.24 Ga-68 PSMA-11 showed a higher sensitivity for detecting index lesions (90.1%) compared with mpMRI (83.1%), although this difference was not significant (p = 0.267). The median size of index tumor foci missed by mpMRI (1.66 cm3; interquartile range [IQR], 0.79–2.53 cm3) was significantly larger than that of tumor foci missed by Ga-68 PSMA-11 PET/CT (0.72 cm3; IQR, 0.36–1.0 cm3; p = 0.034). Among the 136 patients who had clinically significant PCa detected on biopsy (defined as Gleason score of ≥7), Ga-68 PSMA-11 was significantly more sensitive than mpMRI in detecting cancer within the prostate (95% vs. 86%, respectively;p = 0.017), but both imaging methods had high specificities (93% vs. 94%, respectively). Overall, Ga-68 PSMA-11 PET/CT detected significantly more cancer than mpMRI for the entire cohort based on both biopsy (p</i >= 0.004) and RP histopathology (p = 0.020).
In another study evaluating 54 patients with PCa with a median PSA level of 13.30 ng/mL, Ga-68 PSMA-11 PET/CT and PET/MRI were more sensitive in detecting extracapsular extension (PET/CT, 78%; PET/MRI, 83%) compared with mpMRI (mpMRI, 54%;p < 0.05).22 Ga-68 PSMA-11 PET/CT and PET/MRI also tended to have higher sensitivity for detecting seminal vesicle invasion (75%) compared with mpMRI (67%), but the difference was not statistically significant. The specificity, PPV, and NPV were also not significantly different among these modalities. The timing of biopsy (before vs after Ga-68 PSMA-617 PET/CT) did not seem to affect the outcomes of Ga-68 PSMA PET/CT imaging in high-risk patients with PCa.66
Initial PCa N and M staging
In the multicenter proPSMA study, 302 patients were randomly assigned to undergo Ga-68 PSMA-11 PET/CT or conventional imaging (combination of CT and bone scan) for the evaluation of pelvic nodal and distant metastatic disease.37 Patients were included in the study if they had untreated, biopsy-proven PCa; were being considered for curative-intent treatment; and had ≥1 high-risk criterion (PSA ≥20 ng/mL, International Society of Uropathology grade group 3–5, or clinical stage ≥T3). In these patients, Ga-68 PSMA-11 PET/CT demonstrated a 27% (95% confidence interval [CI], 23%–31%; p < 0.0001) absolute greater area under the curve (AUC) for accuracy than conventional imaging (92% vs. 65%, respectively). Conventional imaging, when compared with Ga-68 PSMA-11 PET/CT, had lower sensitivity (38% vs. 85%) and specificity (91% vs. 98%).
In another multicenter trial evaluating 764 patients, Ga-68 PSMA-11 PET/CT or PET/MRI was assessed for its accuracy in detecting pelvic nodal metastases compared with histopathology at the time of RP and pelvic lymph node dissection.63 Patients were included if they had histopathology-proven PCa, were planning to undergo RP, and had intermediate- to high-risk disease (PSA level >10 ng/mL, T-state ≥T2b, Gleason score >6, or other risk factors). The sensitivity and specificity of Ga-68 PSMA-11 PET for pelvic nodal metastases were 40% and 95%, respectively. The sensitivities from this study were lower than the 59% weighted sensitivity reported in a systematic review, although the sensitivities in the systematic review did range from 23% to 100%.67 This large variance in sensitivity and specificity for Ga-68 PSMA-11 across studies is likely explained by differences in study design such as the reference standard used, whether data were collected prospectively or retrospectively, and whether patients were recruited consecutively or nonconsecutively.
Effect of Ga-68 PSMA-11 PET On the initial management of PCa
Six studies (n = 493) reported a change in clinical management with Ga-68 PSMA-11 PET in 19% to 52% of patients with primary PCa.22,35,37,42,60,68 Hofman and colleagues37 reported a significant change in treatment plan in 28% of patients undergoing Ga-68 PSMA-11 PET/CT compared with 15% of patients undergoing conventional imaging (p = 0.0076). Similarly, Wong and colleagues68 evaluated the effect of Ga-68 PSMA-11 PET on disease staging in 131 patients with biopsy-proven PCa. Ga-68 PSMA-11 PET led to a change in PCa stage in 28% of patients, with disease being upstaged in 13% of patients and downstaged in 15% of patients (p < 0.001) when compared with the stage assigned using conventional imaging. These findings suggest that Ga-68 PSMA-11 has the potential to provide more accurate staging for metastatic disease, thereby allowing for more risk-appropriate management through the selection of local vs systemic management. Patients may, therefore, receive more appropriate treatment, although the effects of these changes on cancer-specific and overall survival are yet to be determined.
Detection of BCR
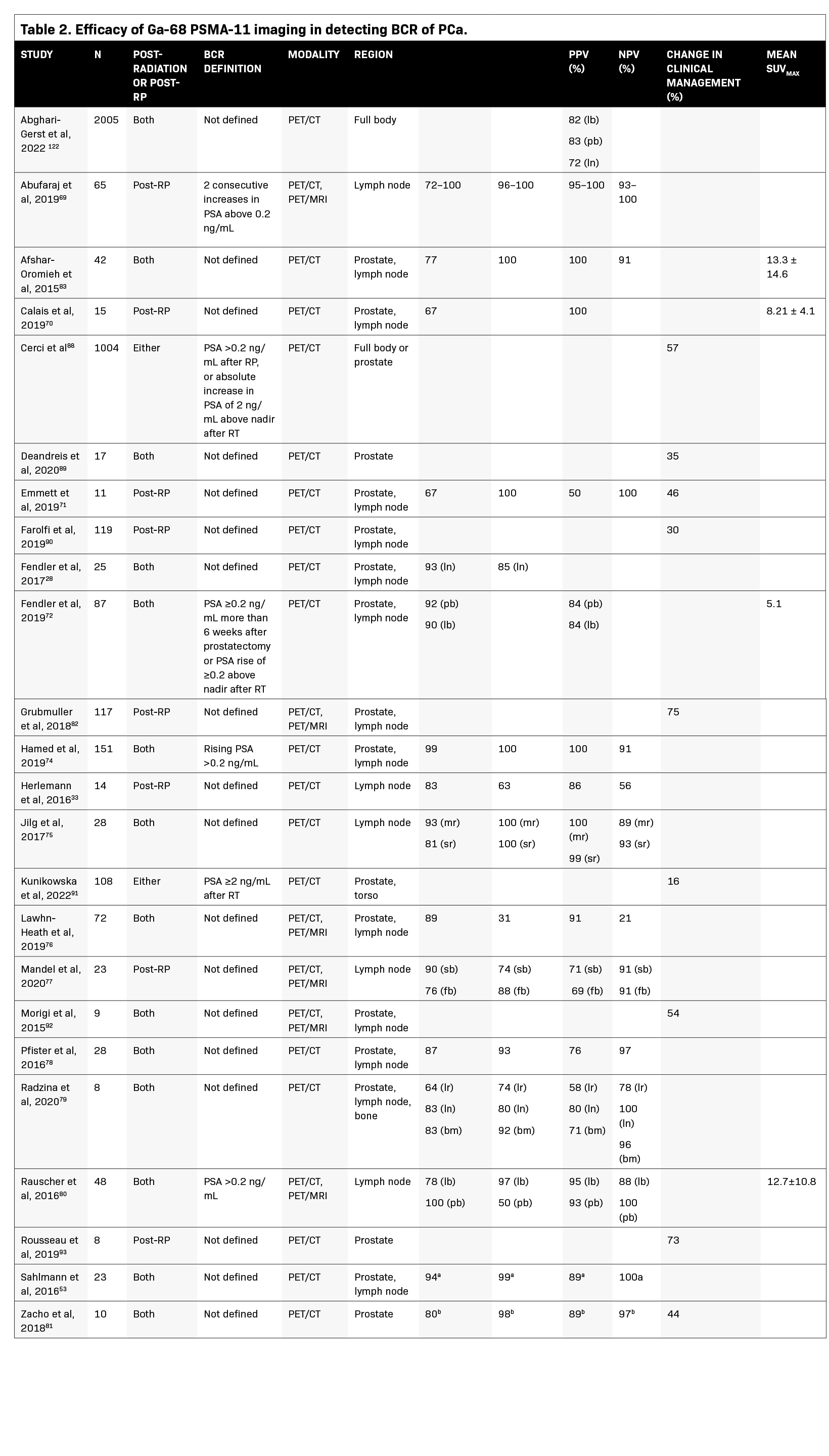
Table 2 summarizes studies that evaluated Ga-68 PSMA-11 PET to assess BCR. The majority of studies demonstrate a sensitivity ≥80% and a specificity ≥90%. Sensitivity values ranged from 64% to 99% for the prostate, 72% to 100% for the lymph nodes, and one study reported a sensitivity of 83% for bone metastases. Specificity values ranged from 31% to 100% for the prostate, 50% to 100% for the lymph nodes, and one study reported a specificity of 92% for bone metastases.28,33,53,69-83 The large variance in sensitivity and specificity can be explained by varying factors across studies, such as areas examined (prostate vs lymph nodes) and the reference standard used (Table 2).
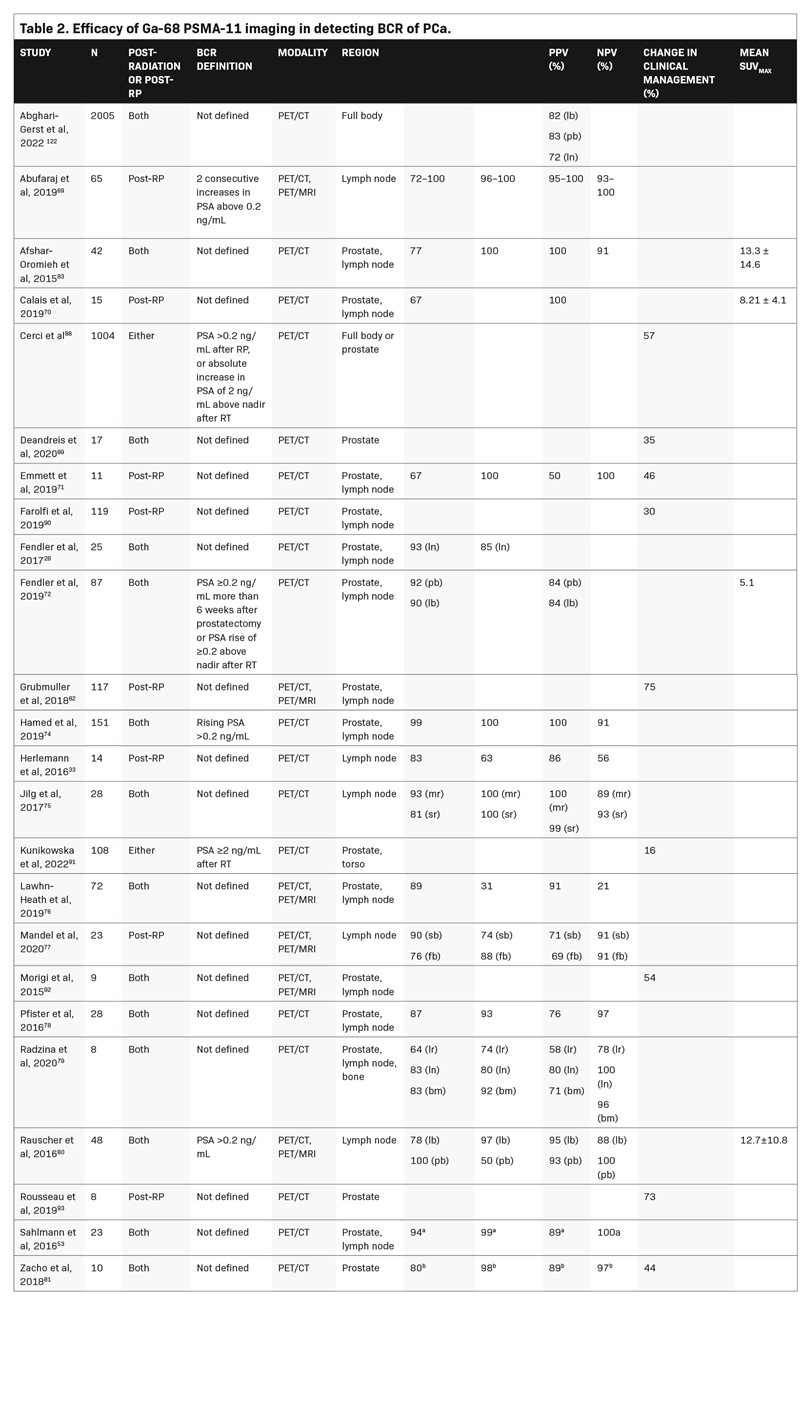
In comparison with conventional imaging, Ga-68 PSMA-11 PET/CT has higher sensitivity, specificity, and accuracy for detecting local recurrence and lymph node metastases, as well as higher detection rates in patients with low PSA levels (≤0.5 ng/mL). In a study of patients who had BCR after definitive PCa treatment with RP (n = 24), radiotherapy (n = 117), or combined treatment (n = 47), patients underwent Ga-68 PSMA-11 PET/CT for the detection of PCa recurrence, with either histologic examination of biopsy sections or 12 months of clinical and imaging follow-up used as the reference standard.74 Ga-68 PSMA-11 PET/CT was found to have a sensitivity of 99% and a specificity of 100% for detecting PCa recurrence. Receiver operating characteristic analysis yielded an ideal PSA cutoff value of >0.65 ng/mL (AUC = 0.964; 95% CI, 0.736–1.000; p < 0.0001), which was associated with a sensitivity of 93% and a specificity of 100% for detecting PCa recurrence with Ga-68 PSMA-11 PET/CT. In patients with lower PSA values (0 to <0.5 ng/ mL), the detection rate was 54.2%.
In a study evaluating 66 patients (median PSA 0.23 ng/mL), Ga-68 PSMA-11 PET/MRI was 55% effective in detecting BCR after RP at low PSA levels (≤0.5 ng/mL), including in patients who had previously undergone or were currently undergoing androgen deprivation therapy (ADT).84 Subgroup analysis of patients with a very low (0 to 0.2 ng/mL) and low PSA (0.2 to 0.5 ng/mL) demonstrated detection rates of 39% and 65%, respectively. Ga-68 PSMA-11 PET/MRI also detected PSMA-positive lesions outside a standard radiotherapy target volume in 39% of patients.
Another study evaluated the accuracy of Ga-68 PSMA-11 PET in detecting lymph node metastases in 65 patients with BCR after RP who were scheduled to undergo salvage lymph node dissection.69 The salvage lymph node dissection templates included lymph nodes from the right and left pelvis, presacral region, and retroperitoneal region. The median diameter of lymph nodes detected on Ga-68 PSMA-11 PET was 7.2 mm (IQR, 5.3–9 mm), whereas the median diameter of false-negative lymph nodes was 3.4 mm (IQR, 2.1–5.4 mm; p = 0.01). Diagnostic accuracy was 99% in the left pelvic region and 95% in the right pelvic, presacral, and retroperitoneal regions. Specificity values were >96% in all regions; sensitivity values were >90% in all but the retroperitoneal region (73%), perhaps because of less dissection of the retroperitoneum during salvage lymph node dissection.
In a study comparing the accuracy of Ga-68 PSMA-11 PET/CT with that of 18F-fluciclovine (a PET radiotracer) PET/CT in detecting BCR after RP in 50 patients, Ga-68 PSMA-11 had a significantly higher detection rate than 18F-fluciclovine (56% vs. 26%, respectively;p = 0.0026).70 Detection rates were significantly higher for Ga-68 PSMA-11 compared with 18F-fluciclovine in the pelvic lymph nodes (30% vs. 8%, respectively;p = 0.0034) and in extrapelvic lesions (16% vs. 0%, respectively;p = 0.0078). Among the 15 patients in whom lesions were verified by histopathology/biopsy, both 18F-fluciclovine and Ga-68 PSMA-11 had PPVs of 100%. Another retrospective analysis of 37 patients with relapsed PCa showed a significantly higher lesion detection rate with Ga-68 PSMA-11 PET/ CT versus standard 18F-fluoromethylcholine PET/CT, especially in patients with low PSA levels.85
Finally, in a pilot study of 14 patients with BCR after RP, 43% of the patients had positive PET scans, including 36% with positive
Ga-68 PSMA-11 scans and 29% with positive 18F-PSMA-1007 scans.86 No additional lesions were identified in the prostate fossa by 18F-PSMA-1007 in comparison to Ga-68 PSMA-11. In a study of 102 patients with BCR, 18F-PSMA-1007 was found to have a significantly higher incidence of PSMA-expressing lesions of benign origin than Ga-68 PSMA-11 (245 vs. 52, respectively).87 Furthermore, the maximum standardized uptake value of these benign lesions was significantly higher (p < 0.0001) for 18F-PSMA-1007, indicating a potentially higher source of false positives with this agent than with Ga-68 PSMA-11.
Effect of Ga-68 PSMA-11 PET On the Management of BCR
Ten studies (n = 1697) reported a change in clinical management with Ga-68 PSMA-11 imaging in 16% to 75% of patients with BCR.71,73,81,82,88-93 In a study of 294 patients, a change in clinical management occurred in 68% of patients, and Ga-68 PSMA-11 PET/CT affected this change in 86% of these patients.73 Treatment modifications guided by Ga-68 PSMA-11 PET/CT were considered effective in 89% of patients; modifications not guided by Ga-68 PSMA-11 PET/CT were considered effective in 61% of patients (p < 0.001). Among patients with BCR following primary curative PCa treatment, delayed imaging with Ga-68 PSMA-11 PET/CT generally led to significantly better uptake and improved contrast, ultimately leading to a change in clinical management for 16% of patients.91 Moreover, in a study of high-risk patients with PCa, primary staging with Ga-68 PSMA-11 PET/CT reduced BCR versus conventional imaging techniques (H = 0.58;p = 0.004).91 Another multicenter prospective trial from 15 countries in 1004 patients with PCa with BCR demonstrated that Ga-68 PSMA-11 PET/CT positivity correlated with Gleason score and PSA level at time of PET scan, PSA doubling time, and radiotherapy as primary treatment. Moreover, treatment modification occurred in 57% of PCa patients with BCR based on the outcomes of Ga-68 PSMA-11 PET/CT imaging.88
Safety Profile of Ga-68 PSMA-11
Ga-68 PSMA-11 is a well-tolerated imaging agent. Five studies (n = 880) reported on the safety of Ga-68 PSMA-11 and found no patients experienced serious adverse events, 18 patients reported experiencing mild adverse events (dizziness, nausea, constipation, diarrhea, headache), and one patient reported a fall after imaging that he attributed to furosemide injection, although there were no associated vital sign changes.12,26,60,72,76 Hofman and colleagues37 also reported a substantially lower radiation exposure with Ga-68 PSMA-11 PET/CT (8.4 mSv) compared with conventional imaging (combination of CT and bone scan) (19.2 mSv;p < 0.001).
Health Economics and Outcomes Research
The cost-effectiveness of Ga-68 PSMA-11 in comparison with conventional imaging has been examined by multiple groups showing that Ga-68 PSMA-11 reduced overall costs because of its increased accuracy in staging, which can obviate the need for unnecessary and costly therapies. In an exploratory analysis evaluating 30 patients over 10 years in Australia, a strategy using Ga-68 PSMA-11 PET/
MRI had an average cost of $39,426 and produced an average of 7.48 years of survival, whereas a strategy involving conventional imaging (bone scan and MRI) had an average cost of $44,667 and produced an average of 7.41 years of survival.94 When the duration of the model was reduced to 5 years, the use of Ga-68 PSMA-11 PET/MRI resulted in cost savings of $3,278 and 0.018 more life-years than conventional imaging. In a cost-effectiveness analysis of the proPSMA study, Ga-68 PSMA-11 PET/CT was found to have a lower estimated cost per scan than the combination of CT and bone scan ($886 vs $1040, respectively).95 In an intention-to-treat analysis evaluating 83 patients with BCR after RP with or without previous radiotherapy, the percentage of patients receiving appropriate curative radio- therapy instead of palliative ADT was 100% with Ga-68 PSMA-11 PET/CT, 74% with C-11 choline PET/CT, and 33% with CT. A retrospective analysis of 244 patients undergoing PSMA PET/CT for recurrent PCa showed that imaging with Ga-68 PSMA-11 was cost-effective compared with 18F-PSMA-1007.96 Outcomes research data are yet to be reported from studies in the United States.
18F-PSMA PET/CT
Although Ga-68 PSMA-11 is mainly used for PET imaging of PCa, other 18F ligands are increasingly becoming available. The US FDA recently approved another PSMA-targeted drug, piflufolastat F-18, for imaging of PCa.97 Similar results were observed with the two agents in other studies in patients with recurrent PCa.92,98,99 In a head-to-head comparison in 16 patients with intermediate/high-risk PCa, Ga-68 PSMA-11 and 18F-PSMA-1007 PET/CT showed similar performance in identifying dominant prostate lesions.100 Another study comparing the 18F-PSMA-1007 PET/CT with Ga-68 PSMA-11 PET/CT in 40 treatment-naïve intermediate/high-risk PCa patients showed comparable detection of primary and metastatic lesions.101
However, defluorination of 18F radiotracers may influence the accuracy of lesion detection in bones due to unspecified bone uptake,102 which can alter the choice of treatment and subsequently affect the quality of life of patients.103 Several recent studies have highlighted that 18F radiotracers are likely to lead to misdiagnosis of bone lesions, with one study reporting nearly 6 times more unspecified bone uptake seen on 18F-PSMA-1007 than with Ga-68 PSMA-11 PET imaging.87,96,104-109 A retrospective analysis of data from 10 patients with PCa who underwent PET-guided biopsy to confirm observations of indeterminate bone lesions on 18F-PSMA-1007 PET/ CT imaging demonstrated that 91% (10/11) of the bone lesions were not metastatic and showed no signs of PSMA expression.110
Another study of 243 patients with high-risk or recurrent PCa reported 98 of 267 bone lesions (37%) in 48 (20%) patients with 18F-DCFPyL PET/CT imaging were indeterminate. Of these indeterminate bone lesions, 37 of 98 (38%) were confirmed benign, 42 of 98 (43%) were malignant, and 19 of 98 (19%) remained equivocal at the lesion level. At the patient level, 24 of 48 (50%) had a benign lesion, 11 of 48 (23%) had a malignant lesion, and 13 of 48 (27%) had equivocal findings.103
A retrospective matched-pair comparison of 18F-rhPSMA-7 with 68-Ga PSMA-11 PET/CT in patients with primary or recurrent PCa showed a higher incidence of benign tumors among PSMA-positive lesions reported with18F-rhPSMA-7 versus 68-Ga PSMA-11 (67% [379/566] vs 35% [100/289]).111
In addition, a study of 283 patients who had 68-Ga PSMA-11 PET and 409 patients who had 18F-PSMA-1007 PET due to BCR showed that 18F-PSMA-1007 PET resulted in a significantly higher rate of nonspecific bone uptake compared with 68-Ga PSMA-11 PET (p < 0.001); however, the rate of bone metastases was not significantly different.109
The updated joint European Association of Nuclear Medicine (EANM) and Society of Nuclear Medicine and Molecular Imaging (SNMMI) procedure guidelines for PCa imaging also note non-specific bone uptake with 18F-rhPSMA-7.3.112 18F PET imaging may also lead to higher interobserver variability, as demonstrated by a retrospective study of 584 patients with newly diagnosed PCa. Significantly increased interobserver variability was observed with 18F-PSMA-1007 for bone metastases versus 18F-DCFPyL and Ga-68 PSMA-11 (p = 0.001 and p = 0.03, respectively), and for overall agreement and locoregional lymph node metastases versus 18F-DCFPyL (p < 0.001 and p = 0.01, respectively).113
Guidelines for PSMA imaging
The updated National Comprehensive Cancer Network guidelines now include guidance regarding the use of Ga-68 PSMA-11.114 The guidelines state that, because of the increased sensitivity and specificity of PSMA PET tracers for detecting micrometastatic disease at initial staging and in cases of BCR, conventional imaging is no longer considered a necessary prerequisite to PSMA PET, and PSMA PET/CT or PSMA PET/MRI can serve as an equally effective or more effective first-line imaging tool for these patients.114 The updated joint EANM and SNMMI procedure guidelines for PCa imaging also include the use of Ga-68 PSMA-11 PET/CT and recommend combining PSMA-PET/CT with multiparametric MRI for guiding biopsy for confirmation of PCa.112 Recently,the Society of Nuclear Medicine and Molecular Imaging, American College of Nuclear Medicine, American Urological Association, Australia and New Zealand Society of Nuclear Medicine, American Society of Clinical Oncology, EANM, and the American College of Physicians worked collaboratively to develop appropriate use criteria for PSMA PET imaging (Table 3).115 In addition, the EANM criteria, PROMISE criteria, and PSMA-RADS have also been published to streamline the interpretation of PSMA PET imaging.116
Discussion
Ga-68 PSMA-11 PET/CT is effective in the initial staging and detection of PCa BCR and has advantages over MRI in the initial local staging of PCa, mainly detection of extraprostatic disease in initial staging and BCR and at low PSA levels (≤0.5 ng/mL); potential for leading to a change in radiotherapy target planning84; and cost-effectiveness while reducing the amount of radiation exposure to the patient.94 Bone lesions are easier to interpret on Ga-68 PSMA-11 compared to 18F-based radiotracer imaging.87,104,111,112 Ga-68 PSMA-11 is well tolerated, further supporting its potential as the imaging agent of choice in PCa.
Ga-68 PSMA-11 has also been used for confirming primary or recurrent PCa in several studies, demonstrating its diagnostic value in clinical practice. Ga-68 PSMA-11 PET/CT was used in combination with MRI to triage patients for biopsy during initial diagnosis and improved NPV for ruling out clinically significant PCa, thereby reducing the number of unnecessary biopsies.117 In addition, Ga-68 PSMA-11 PET/CT was useful for guiding metastasis-directed radiotherapy in patients with oligometastatic PCa recurrence, delaying the need for ADT and potentially prolonging BCR-free survival.118
In a study of patients with metastatic castration-resistant PCa, Ga-68 PSMA-11 PET provided reliable parameters that could be used to predict response to systemic therapies.119 68-Ga PSMA-11 was also used for confirmation of metastatic castration-resistant PCa and identification of appropriate patients for PSMA-based radioligand therapy in the phase 3 VISION trial,120 and is approved in the US for patient selection for PSMA-directed radioligand therapy. Finally, Ga-68 PSMA-11 PET/CT may also be useful in determining appropriate candidates for RP, as the technique has high PPV and specificity for identifying lymph node metastases and local recurrence.121
The main limitation of this review is the heterogeneity of the included studies (varying sample sizes, patients being grouped by differing PSA ranges). Variations in reported diagnostic accuracy parameters were seen as anticipated given differences in patient characteristics (eg, PSA, lesion sizes) and study designs. Also, additional studies are needed to determine the effects of Ga-68 PSMA-11 on cost.
In summary, Ga-68 PSMA-11 PET has a favorable safety profile that affords high accuracy for PCa initial staging and the detection of PCa BCR. Although more studies are needed, its use frequently leads to changes in treatment that may positively affect patient outcomes. With increased access, the use of Ga-68 PSMA-11 is expected to expand and include additional applications.
References
- Sung H, Ferlay J, RL Siegel, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
- Siegel RL, KD Miller, NS Wagle, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48.
- Siegel DA, ME O’Neil, TB Richards, et al. Prostate cancer incidence and survival, by stage and race/ethnicity - United States, 2001-2017. MMWR Morb Mortal Wkly Rep. 2020;69(41):1473-1480.
- Lowrance WT, RH Breau, R Chou, et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J Urol. 2021;205(1):14-21.
- Atan A and Ö Güzel, How should prostate specific antigen be interpreted? Turk J Urol. 2013;39(3):188-93.
- Venkatesan AM, E Mudairu-Dawodu, C Duran, et al. Detecting recurrent prostate Cancer using multiparametric MRI, influence of PSA and Gleason grade. Cancer Imaging. 2021;21(1):3.
- Dess RT, TM Morgan, PL Nguyen, et al. Adjuvant Versus Early Salvage Radiation Therapy Following Radical Prostatectomy for Men with Localized Prostate Cancer. Curr Urol Rep. 2017;18(7):55.
- Barakzai MA, Prostatic Adenocarcinoma: A Grading from Gleason to the New Grade-Group System: A Historical and Critical Review. Asian Pac J Cancer Prev. 2019;20(3):661-666.
- Borley N and MR Feneley, Prostate cancer: diagnosis and staging. Asian J Androl. 2009;11(1):74-80.
- Spratt DE, DJ McHugh, MJ Morris, et al. Management of Biochemically Recurrent Prostate Cancer: Ensuring the Right Treatment of the Right Patient at the Right Time. Am Soc Clin Oncol Educ Book. 2018;38:355-362.
- Broderick JM, NCCN Guidelines add PSMA-PET imaging modalities for prostate cancer. Urology Times. 2021.
- Hicks RM, JP Simko, AC Westphalen, et al. Diagnostic Accuracy of (68)Ga-PSMA-11 PET/MRI Compared with Multiparametric MRI in the Detection of Prostate Cancer. Radiology. 2018;289(3):730-737.
- Banerjee SR and MG Pomper, Clinical applications of Gallium-68. Appl Radiat Isot. 2013;76:2-13.
- Carlucci G, R Ippisch, R Slavik, et al. (68)Ga-PSMA-11 NDA Approval: A Novel and Successful Academic Partnership. J Nucl Med. 2021;62(2):149-155.
- Telix Pharmaceuticals Limited, FDA Approves Telix’s Prostate Cancer Imaging Product, Illuccix®. 2021, Telix Pharmaceuticals Limited: Melbourne, Australia-Indianapolis, IN.
- Sonni I, M Eiber, WP Fendler, et al. Impact of (68)Ga-PSMA-11 PET/CT on Staging and Management of Prostate Cancer Patients in Various Clinical Settings: A Prospective Single-Center Study. J Nucl Med. 2020;61(8):1153-1160.
- Al-Bayati M, J Grueneisen, S Lütje, et al. Integrated 68Gallium Labelled Prostate-Specific Membrane Antigen-11 Positron Emission Tomography/Magnetic Resonance Imaging Enhances Discriminatory Power of Multi-Parametric Prostate Magnetic Resonance Imaging. Urol Int. 2018;100(2):164-171.
- Basha MAA, MAG Hamed, O Hussein, et al. (68)Ga-PSMA-11 PET/CT in newly diagnosed prostate cancer: diagnostic sensitivity and interobserver agreement. Abdom Radiol (NY). 2019;44(7):2545-2556.
- Berger I, C Annabattula, J Lewis, et al. (68)Ga-PSMA PET/CT vs. mpMRI for locoregional prostate cancer staging: correlation with final histopathology. Prostate Cancer Prostatic Dis. 2018;21(2):204-211.
- Budäus L, SR Leyh-Bannurah, G Salomon, et al. Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur Urol. 2016;69(3):393-6.
- Chen M, X Qiu, Q Zhang, et al. PSMA uptake on [68Ga]-PSMA-11-PET/CT positively correlates with prostate cancer aggressiveness. Q J Nucl Med Mol Imaging. 2022;66(1):67-73.
- Chen M, Q Zhang, C Zhang, et al. Comparison of (68)Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) and multi-parametric magnetic resonance imaging (MRI) in the evaluation of tumor extension of primary prostate cancer. Transl Androl Urol. 2020;9(2):382-390.
- Demirci E, L Kabasakal, OE Şahin, et al. Can SUVmax values of Ga-68-PSMA PET/CT scan predict the clinically significant prostate cancer? Nucl Med Commun. 2019;40(1):86-91.
- Donato P, A Morton, J Yaxley, et al. (68)Ga-PSMA PET/CT better characterises localised prostate cancer after MRI and transperineal prostate biopsy: Is (68)Ga-PSMA PET/CT guided biopsy the future? Eur J Nucl Med Mol Imaging. 2020;47(8):1843-1851.
- Donato P, MJ Roberts, A Morton, et al. Improved specificity with (68)Ga PSMA PET/CT to detect clinically significant lesions “invisible” on multiparametric MRI of the prostate: a single institution comparative analysis with radical prostatectomy histology. Eur J Nucl Med Mol Imaging. 2019;46(1):20-30.
- El Hajj A, B Yacoub, M Mansour, et al. Diagnostic performance of Gallium-68 prostate-specific membrane antigen positron emission tomography-computed tomography in intermediate and high risk prostate cancer. Medicine (Baltimore). 2019;98(44):e17491.
- Fendler WP, DF Schmidt, V Wenter, et al. 68Ga-PSMA PET/CT Detects the Location and Extent of Primary Prostate Cancer. J Nucl Med. 2016;57(11):1720-1725.
- 2Fendler WP, J Calais, M Allen-Auerbach, et al. (68)Ga-PSMA-11 PET/CT Interobserver Agreement for Prostate Cancer Assessments: An International Multicenter Prospective Study. J Nucl Med. 2017;58(10):1617-1623.
- Ferraro DA, UJ Muehlematter, HI Garcia Schüler, et al. (68)Ga-PSMA-11 PET has the potential to improve patient selection for extended pelvic lymph node dissection in intermediate to high-risk prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47(1):147-159.
- Gao J, C Zhang, Q Zhang, et al. Diagnostic performance of (68)Ga-PSMA PET/CT for identification of aggressive cribriform morphology in prostate cancer with whole-mount sections. Eur J Nucl Med Mol Imaging. 2019;46(7):1531-1541.
- Gupta M, PS Choudhury, D Hazarika, et al. A Comparative Study of (68)Gallium-Prostate Specific Membrane Antigen Positron Emission Tomography-Computed Tomography and Magnetic Resonance Imaging for Lymph Node Staging in High Risk Prostate Cancer Patients: An Initial Experience. World J Nucl Med. 2017;16(3):186-191.
- Gupta M, PS Choudhury, S Rawal, et al. Initial risk stratification and staging in prostate cancer with prostatic-specific membrane antigen positron emission tomography/computed tomography: A first-stop-shop. World J Nucl Med. 2018;17(4):261-269.
- Herlemann A, V Wenter, A Kretschmer, et al. (68)Ga-PSMA Positron Emission Tomography/Computed Tomography Provides Accurate Staging of Lymph Node Re- gions Prior to Lymph Node Dissection in Patients with Prostate Cancer. Eur Urol. 2016;70(4):553-557.
- Hinsenveld FJ, EMK Wit, PJ van Leeuwen, et al. Prostate-Specific Membrane Antigen PET/CT Combined with Sentinel Node Biopsy for Primary Lymph Node Staging in Prostate Cancer. J Nucl Med. 2020;61(4):540-545.
- Hirmas N, A Al-Ibraheem, K Herrmann, et al. [(68)Ga]PSMA PET/CT Improves Initial Staging and Management Plan of Patients with High-Risk Prostate Cancer. Mol Imaging Biol. 2019;21(3):574-581.
- 3Hoffmann MA, M Miederer, HJ Wieler, et al. Diagnostic performance of (68)Gallium-PSMA-11 PET/CT to detect significant prostate cancer and comparison with (18) FEC PET/CT. Oncotarget. 2017;8(67):111073-111083.
- 3Hofman MS, N Lawrentschuk, RJ Francis, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208-1216.
- Jena A, R Taneja, S Taneja, et al. Improving Diagnosis of Primary Prostate Cancer With Combined (68)Ga-Prostate-Specific Membrane Antigen-HBED-CC Simultaneous PET and Multiparametric MRI and Clinical Parameters. AJR Am J Roentgenol. 2018;211(6):1246-1253.
- 3Kalapara AA, T Nzenza, HYC Pan, et al. Detection and localisation of primary prostate cancer using (68) gallium prostate-specific membrane antigen positron emission tomography/computed tomography compared with multiparametric magnetic resonance imaging and radical prostatectomy specimen pathology. BJU Int. 2020;126(1):83-90.
- Kopp J, D Kopp, E Bernhardt, et al. (68)Ga-PSMA PET/CT based primary staging and histological correlation after extended pelvic lymph node dissection at radical prostatectomy. World J Urol. 2020;38(12):3085-3090.
- Liu C, T Liu, Z Zhang, et al. (68)Ga-PSMA PET/CT Combined with PET/Ultrasound-Guided Prostate Biopsy Can Diagnose Clinically Significant Prostate Cancer in Men with Previous Negative Biopsy Results. J Nucl Med. 2020;61(9):1314-1319.
- Lopci E, G Lughezzani, A Castello, et al. Prospective Evaluation of (68)Ga-labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Com- puted Tomography in Primary Prostate Cancer Diagnosis. Eur Urol Focus. 2021;7(4):764-771.
- Lopci E, A Saita, M Lazzeri, et al. (68)Ga-PSMA Positron Emission Tomography/Computerized Tomography for Primary Diagnosis of Prostate Cancer in Men with Contraindications to or Negative Multiparametric Magnetic Resonance Imaging: A Prospective Observational Study. J Urol. 2018;200(1):95-103.
- Maurer T, JE Gschwend, I Rauscher, et al. Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J Urol. 2016;195(5):1436-1443.
- Muehlematter UJ, IA Burger, AS Becker, et al. Diagnostic Accuracy of Multiparametric MRI versus (68)Ga-PSMA-11 PET/MRI for Extracapsular Extension and Seminal Vesicle Invasion in Patients with Prostate Cancer. Radiology. 2019;293(2):350-358.
- Nandurkar R, P van Leeuwen, P Stricker, et al. (68)Ga-HBEDD PSMA-11 PET/CT staging prior to radical prostatectomy in prostate cancer patients: Diagnostic and predictive value for the biochemical response to surgery. Br J Radiol. 2019;92(1095):20180667.
- Öbek C, T Doğanca, E Demirci, et al. The accuracy of (68)Ga-PSMA PET/CT in primary lymph node staging in high-risk prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44(11):1806-1812.
- Pallavi UN, S Gogoi, P Thakral, et al. Incremental value of Ga-68 prostate-specific membrane antigen-11 positron-emission tomography/computed tomography scan for preoperative risk stratification of prostate cancer. Indian J Nucl Med. 2020;35(2):93-99.
- Park SY, C Zacharias, C Harrison, et al. Gallium 68 PSMA-11 PET/MR Imaging in Patients with Intermediate- or High-Risk Prostate Cancer. Radiology. 2018;288(2):495-505.
- Petersen LJ, JB Nielsen, NC Langkilde, et al. (68)Ga-PSMA PET/CT compared with MRI/CT and diffusion-weighted MRI for primary lymph node staging prior to definitive radiotherapy in prostate cancer: a prospective diagnostic test accuracy study. World J Urol. 2020;38(4):939-948.
- Rahbar K, M Weckesser, S Huss, et al. Correlation of Intraprostatic Tumor Extent with ⁶⁸Ga-PSMA Distribution in Patients with Prostate Cancer. J Nucl Med. 2016;57(4):563-7.
- Rahman LA, D Rutagengwa, P Lin, et al. High negative predictive value of 68Ga PSMA PET-CT for local lymph node metastases in high risk primary prostate cancer with histopathological correlation. Cancer Imaging. 2019;19(1):86.
- Sahlmann CO, B Meller, C Bouter, et al. Biphasic ⁶⁸Ga-PSMA-HBED-CC-PET/CT in patients with recurrent and high-risk prostate carcinoma. Eur J Nucl Med Mol Imag- ing. 2016;43(5):898-905.
- Thalgott M, C Düwel, I Rauscher, et al. One-stop-shop whole-body (68)Ga-PSMA-11 PET/MRI compared with clinical nomograms for preoperative T and n staging of high-risk prostate cancer. J Nucl Med. 2018;59(12):1850-1856.
- 5\Tulsyan S, CJ Das, M Tripathi, et al. Comparison of 68Ga-PSMA PET/CT and multiparametric MRI for staging of high-risk prostate cancer68Ga-PSMA PET and MRI in prostate cancer. Nucl Med Commun. 2017;38(12):1094-1102.
- \van Leeuwen PJ, L Emmett, B Ho, et al. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomogra- phy for preoperative lymph node staging in prostate cancer. BJU Int. 2017;119(2):209-215.
- \van Leeuwen PJ, M Donswijk, R Nandurkar, et al. Gallium-68-prostate-specific membrane antigen ((68) Ga-PSMA) positron emission tomography (PET)/computed tomography (CT) predicts complete biochemical response from radical prostatectomy and lymph node dissection in intermediate- and high-risk prostate cancer. BJU Int. 2019;124(1):62-68.
- \von Klot CJ, AS Merseburger, A Böker, et al. (68)Ga-PSMA PET/CT imaging predicting intraprostatic tumor extent, extracapsular extension and seminal vesicle invasion prior to radical prostatectomy in patients with prostate cancer. Nucl Med Mol Imaging. 2017;51(4):314-322.
- Yilmaz B, R Turkay, Y Colakoglu, et al. Comparison of preoperative locoregional Ga-68 PSMA-11 PET-CT and mp-MRI results with postoperative histopathology of prostate cancer. Prostate. 2019;79(9):1007-1017.
- Zang S, G Shao, C Cui, et al. 68Ga-PSMA-11 PET/CT for prostate cancer staging and risk stratification in Chinese patients. Oncotarget. 2017;8(7):12247-12258.
- Zhang Q, S Zang, C Zhang, et al. Comparison of (68)Ga-PSMA-11 PET-CT with mpMRI for preoperative lymph node staging in patients with intermediate to high-risk prostate cancer. J Transl Med. 2017;15(1):230.
- Zhang J, S Shao, P Wu, et al. Diagnostic performance of (68)Ga-PSMA PET/CT in the detection of prostate cancer prior to initial biopsy: comparison with cancer-predicting nomograms. Eur J Nucl Med Mol Imaging. 2019;46(4):908-920.
- Hope TA, M Eiber, WR Armstrong, et al. Diagnostic accuracy of 68Ga-PSMA-11 pet for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: A multicenter prospective phase 3 imaging trial. JAMA Oncol. 2021;7(11):1635-1642.
- Yaxley JW, S Raveenthiran, FX Nouhaud, et al. Outcomes of primary lymph node staging of intermediate and high risk prostate cancer with (68)Ga-PSMA positron emission tomography/computerized tomography compared to histological correlation of pelvic lymph node pathology. J Urol. 2019;201(4):815-820.
- Klingenberg S, J Fredsøe, KD Sørensen, et al. Recurrence rate after radical prostatectomy following primary staging of high-risk prostate cancer with (68)Ga-PSMA PET/CT. Acta Oncol. 2022;61(10):1289-1294.
- Zou S, S Song, J Zhou, et al. Time point-independent tumor positivity of (68)Ga-PSMA-PET/CT pre- and post-biopsy in high-risk prostate cancer. Ann Nucl Med. 2022;36(6):523-532.
- Petersen LJ and HD Zacho, PSMA PET for primary lymph node staging of intermediate and high-risk prostate cancer: an expedited systematic review. Cancer Imaging. 2020;20(1):10.
- Wong HS, J Leung, D Bartholomeusz, et al. Comparative study between (68) Ga-prostate-specific membrane antigen positron emission tomography and conventional imaging in the initial staging of prostate cancer. J Med Imaging Radiat Oncol. 2018;62(6):816-822.
- Abufaraj M, B Grubmüller, M Zeitlinger, et al. Prospective evaluation of the performance of [(68)Ga]Ga-PSMA-11 PET/CT(MRI) for lymph node staging in patients undergoing superextended salvage lymph node dissection after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2019;46(10):2169-2177.
- Calais J, F Ceci, M Eiber, et al. (18)F-fluciclovine PET-CT and (68)Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20(9):1286-1294.
- Emmett L, U Metser, G Bauman, et al. Prospective, multisite, international comparison of (18)F-Fluoromethylcholine PET/CT, multiparametric MRI, and (68)Ga-HBED-CC PSMA-11 PET/CT in men with high-risk features and biochemical failure after radical prostatectomy: Clinical performance and patient outcomes. J Nucl Med. 2019;60(6):794-800.
- Fendler WP, J Calais, M Eiber, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: A prospective single-arm clinical trial. JAMA Oncol. 2019;5(6):856-863.
- Fourquet A, L Lahmi, T Rusu, et al. Restaging the biochemical recurrence of prostate cancer with [(68)Ga]Ga-PSMA-11 PET/CT: Diagnostic performance and impact on patient disease management. Cancers (Basel). 2021;13(7).
- Hamed MAG, MAA Basha, H Ahmed, et al. (68)Ga-PSMA PET/CT in patients with rising prostatic-specific antigen after definitive treatment of prostate cancer: Detection efficacy and diagnostic accuracy. Acad Radiol. 2019;26(4):450-460.
- Jilg CA, V Drendel, HC Rischke, et al. Diagnostic Accuracy of Ga-68-HBED-CC-PSMA-Ligand-PET/CT before Salvage Lymph Node Dissection for Recurrent Prostate Cancer. Theranostics. 2017;7(6):1770-1780.
- Lawhn-Heath C, RR Flavell, SC Behr, et al. Single-center prospective evaluation of (68)Ga-PSMA-11 PET in biochemical recurrence of prostate cancer. AJR Am J Roent- genol. 2019;213(2):266-274.
- Mandel P, D Tilki, FK Chun, et al. Accuracy of (68)ga-prostate-specific membrane antigen positron emission tomography for the detection of lymph node metastases before salvage lymphadenectomy. Eur Urol Focus. 2020;6(1):71-73.
- Pfister D, D Porres, A Heidenreich, et al. Detection of recurrent prostate cancer lesions before salvage lymphadenectomy is more accurate with (68)Ga-PSMA-HBED- CC than with (18)F-Fluoroethylcholine PET/CT. Eur J Nucl Med Mol Imaging. 2016;43(8):1410-7.
- Radzina M, M Tirane, L Roznere, et al. Accuracy of (68)Ga-PSMA-11 PET/CT and multiparametric MRI for the detection of local tumor and lymph node metastases in early biochemical recurrence of prostate cancer. Am J Nucl Med Mol Imaging. 2020;10(2):106-118.
- Rauscher I, T Maurer, AJ Beer, et al. Value of 68Ga-PSMA HBED-CC PET for the Assessment of Lymph Node Metastases in Prostate Cancer Patients with Biochemical Recurrence: Comparison with Histopathology After Salvage Lymphadenectomy. J Nucl Med. 2016;57(11):1713-1719.
- Zacho HD, JB Nielsen, K Dettmann, et al. 68Ga-PSMA PET/CT in patients with biochemical recurrence of prostate cancer: A prospective, 2-center study. Clin Nucl Med. 2018;43(8):579-585.
- Grubmüller B, P Baltzer, D D’Andrea, et al. (68)Ga-PSMA 11 ligand PET imaging in patients with biochemical recurrence after radical prostatectomy - diagnostic performance and impact on therapeutic decision-making. Eur J Nucl Med Mol Imaging. 2018;45(2):235-242.
- Afshar-Oromieh A, E Avtzi, FL Giesel, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(2):197-209.
- Kranzbühler B, J Müller, AS Becker, et al. Detection Rate and Localization of Prostate Cancer Recurrence Using (68)Ga-PSMA-11 PET/MRI in Patients with Low PSA Values ≤ 0.5 ng/mL J Nucl Med. 2020;61(2):194-201.
- Afshar-Oromieh A, CM Zechmann, A Malcher, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagno- sis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41(1):11-20.
- Sheehan-Dare G, J Ende, A Amin, et al. Pilot trial comparing the performance of 68Ga-PSMA-11 PET/CT to 18F-PSMA-1007 PET/CT in the detection of prostate cancer recurrence in men with rising PSA following radical prostatectomy. Journal of Nuclear Medicine. 2021;62(supplement 1):1323-1323.
- Rauscher I, M Krönke, M König, et al. Matched-Pair Comparison of (68)Ga-PSMA-11 PET/CT and (18)F-PSMA-1007 PET/CT: Frequency of Pitfalls and Detection Efficacy in Biochemical Recurrence After Radical Prostatectomy. J Nucl Med. 2020;61(1):51-57.
- Cerci JJ, S Fanti, EE Lobato, et al. Diagnostic performance and clinical impact of (68)Ga-PSMA-11 PET/CT imaging in early relapsed prostate cancer after radical therapy: A prospective multicenter study (IAEA-PSMA Study). J Nucl Med. 2022;63(2):240-247.
- Deandreis D, A Guarneri, F Ceci, et al. (68)Ga-PSMA-11 PET/CT in recurrent hormone-sensitive prostate cancer (HSPC): a prospective single-centre study in patients eligible for salvage therapy. Eur J Nucl Med Mol Imaging. 2020;47(12):2804-2815.
- Farolfi A, F Ceci, P Castellucci, et al. (68)Ga-PSMA-11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA <0.5 ng/ ml. Efficacy and impact on treatment strategy. Eur J Nucl Med Mol Imaging. 2019;46(1):11-19.
- Kunikowska J, K Pełka, O Tayara, et al. Ga-68-PSMA-11 PET/CT in patients with biochemical recurrence of prostate cancer after primary treatment with curative intent-impact of delayed imaging. J Clin Med. 2022;11(12).
- Morigi JJ, PD Stricker, PJ van Leeuwen, et al. Prospective comparison of 18F-fluoromethylcholine Versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56(8):1185-90.
- Rousseau C, M Le Thiec, L Ferrer, et al. Preliminary results of a (68) Ga-PSMA PET/CT prospective study in prostate cancer patients with occult recurrence: Diagnostic performance and impact on therapeutic decision-making. Prostate. 2019;79(13):1514-1522.
- Gordon LG, TM Elliott, A Joshi, et al. Exploratory cost-effectiveness analysis of (68)Gallium-PSMA PET/MRI-based imaging in patients with biochemical recurrence of prostate cancer. Clin Exp Metastasis. 2020;37(2):305-312.
- de Feria Cardet RE, MS Hofman, T Segard, et al. Is prostate-specific membrane antigen positron emission tomography/computed tomography imaging cost-effective in prostate cancer: An analysis informed by the proPSMA trial. Eur Urol. 2021;79(3):413-418.
- Alberts I, C Mingels, HD Zacho, et al. Comparing the clinical performance and cost efficacy of [68Ga]Ga-PSMA-11 and [18}F]PSMA-1007 in the diagnosis of recurrent prostate cancer: a Markov chain decision analysis. Eur J Nucl Med Mol Imaging. 2022;49(12):4252-4261.
- Keam SJ, Piflufolastat F 18: Diagnostic first approval. Mol Diagn Ther. 2021;25(5):647-656.
- Paymani Z, T Rohringer, R Vali, et al. Diagnostic performance of [(18)F]fluorocholine and [(68)Ga]Ga-PSMA PET/CT in prostate cancer: A comparative study. J Clin Med. 2020;9(7).
- Calais J, WP Fendler, K Herrmann, et al. Comparison of (68)Ga-PSMA-11 and (18)F-Fluciclovine PET/CT in a case series of 10 patients with prostate cancer recurrence. J Nucl Med. 2018;59(5):789-794.
- Kuten J, I Fahoum, Z Savin, et al. Head-to-head comparison of (68)Ga-PSMA-11 with (18)F-PSMA-1007 PET/CT in staging prostate cancer using histopathology and immunohistochemical analysis as a reference standard. J Nucl Med. 2020;61(4):527-532.
- Chandekar KR, H Singh, R Kumar, et al. Comparison of 18 F-PSMA-1007 PET/CT With 68 Ga-PSMA-11 PET/CT for Initial Staging in Intermediate- and High-Risk Prostate Cancer. Clin Nucl Med. 2023;48(1):e1-e8.
- Lütje S, GM Franssen, K Herrmann, et al. In vitro and in vivo characterization of an (18)F-AlF-labeled PSMA ligand for imaging of PSMA-expressing xenografts. J Nucl Med. 2019;60(7):1017-1022.
- Phelps TE, SA Harmon, E Mena, et al. Predicting Outcomes of Indeterminate Bone Lesions on (18)F-DCFPyL PSMA PET/CT Scans in the Setting of High-Risk Primary or Recurrent Prostate Cancer. J Nucl Med. 2022.
- Grünig H, A Maurer, Y Thali, et al. Focal unspecific bone uptake on [(18)F]-PSMA-1007 PET: a multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. Eur J Nucl Med Mol Imaging. 2021;48(13):4483-4494.
- Wondergem M, FM van der Zant, WAM Broos, et al. Matched-pair comparison of (18)F-DCFPyL PET/CT and (18)F-PSMA-1007 PET/CT in 240 prostate cancer patients: Interreader agreement and lesion detection rate of suspected lesions. J Nucl Med. 2021;62(10):1422-1429.
- Hoberück S, S Löck, A Borkowetz, et al. Intraindividual comparison of [(68) Ga]-Ga-PSMA-11 and [(18)F]-F-PSMA-1007 in prostate cancer patients: a retrospective single-center analysis. EJNMMI Res. 2021;11(1):109.
- Hammes J, M Hohberg, P Täger, et al. Uptake in non-affected bone tissue does not differ between [18F]-DCFPyL and [68Ga]-HBED-CC PSMA PET/CT. PLoS One. 2018;13(12):e0209613.
- Byrne M, N Ranasinha, C Mercader, et al. Fluorine-18 (18F) prostate-specific membrane antigen (PSMA) positron emission tomography (PET) and the diagnosis and staging of primary prostate cancer (PCa). in AUA 2022. 2022. New Orleans, LA: May 13-16, 2022.
- Seifert R, T Telli, M Opitz, et al. Unspecific (18)F-PSMA-1007 bone uptake evaluated through PSMA-11 PET, bone scanning, and MRI triple validation in patients with biochemical recurrence of prostate cancer. J Nucl Med. 2023;64(5):738-743.
- Vollnberg B, I Alberts, V Genitsch, et al. Assessment of malignancy and PSMA expression of uncertain bone foci in [(18)F]PSMA-1007 PET/CT for prostate cancer-a single-centre experience of PET-guided biopsies. Eur J Nucl Med Mol Imaging. 2022;49(11):3910-3916.
- Kroenke M, L Mirzoyan, T Horn, et al. Matched-pair comparison of (68)Ga-PSMA-11 and (18)F-rhPSMA-7 PET/CT in patients with primary and biochemical recurrence of prostate cancer: Frequency of non-tumor-related uptake and tumor positivity. J Nucl Med. 2021;62(8):1082-1088.
- Fendler WP, M Eiber, M Beheshti, et al. PSMA PET/CT: joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur J Nucl Med Mol Imaging. 2023.
- Hagens MJ, DE Oprea-Lager, AN Vis, et al. Reproducibility of PSMA PET/CT Imaging for Primary Staging of Treatment-Naïve Prostate Cancer Patients Depends on the Applied Radiotracer: A Retrospective Study. J Nucl Med. 2022;63(10):1531-1536.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Prostate Cancer. Version 1.2023.Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf 2022.[cited 2023 09/16/2022]
- Jadvar H, J Calais, S Fanti, et al. Appropriate use criteria for prostate-specific membrane antigen PET imaging. J Nucl Med. 2022;63(1):59-68.
- Ceci F, DE Oprea-Lager, L Emmett, et al. E-PSMA: the EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur J Nucl Med Mol Imaging. 2021;48(5):1626-1638.
- Emmett L, J Buteau, N Papa, et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): A prospective multicentre study. Eur Urol. 2021;80(6):682-689.
- Artigas C, P Flamen, F Charlier, et al. (68)Ga-PSMA PET/CT-based metastasis-directed radiotherapy for oligometastatic prostate cancer recurrence after radical prostatectomy. World J Urol. 2019;37(8):1535-1542.
- Grubmüller B, S Rasul, P Baltzer, et al. Response assessment using [(68) Ga]Ga-PSMA ligand PET in patients undergoing systemic therapy for metastatic castration-resistant prostate cancer. Prostate. 2020;80(1):74-82.
- Sartor O, J de Bono, KN Chi, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021.
- Pfister D, F Haidl, T Nestler, et al. (68) Ga-PSMA-PET/CT helps to select patients for salvage radical prostatectomy with local recurrence after primary radiotherapy for prostate cancer. BJU Int. 2020;126(6):679-683.
- Abghari-Gerst M, WR Armstrong, K Nguyen, et al. A Comprehensive Assessment of (68)Ga-PSMA-11 PET in Biochemically Recurrent Prostate Cancer: Results from a Prospective Multicenter Study on 2,005 Patients. J Nucl Med. 2022;63(4):567-572.
- Fendler WP, M Eiber, M Beheshti, et al. (68)Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44(6):1014-1024
